The molecular biology and immune control of chronic Toxoplasma gondii infection
- PMID: 32609097
- PMCID: PMC7324197
- DOI: 10.1172/JCI136226
The molecular biology and immune control of chronic Toxoplasma gondii infection
Abstract
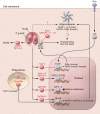
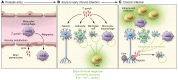
Toxoplasma gondii is an incredibly successful parasite owing in part to its ability to persist within cells for the life of the host. Remarkably, at least 350 host species of T. gondii have been described to date, and it is estimated that 30% of the global human population is chronically infected. The importance of T. gondii in human health was made clear with the first reports of congenital toxoplasmosis in the 1940s. However, the AIDS crisis in the 1980s revealed the prevalence of chronic infection, as patients presented with reactivated chronic toxoplasmosis, underscoring the importance of an intact immune system for parasite control. In the last 40 years, there has been tremendous progress toward understanding the biology of T. gondii infection using rodent models, human cell experimental systems, and clinical data. However, there are still major holes in our understanding of T. gondii biology, including the genes controlling parasite development, the mechanisms of cell-intrinsic immunity to T. gondii in the brain and muscle, and the long-term effects of infection on host homeostasis. The need to better understand the biology of chronic infection is underscored by the recent rise in ocular disease associated with emerging haplotypes of T. gondii and our lack of effective treatments to sterilize chronic infection. This Review discusses the cell types and molecular mediators, both host and parasite, that facilitate persistent T. gondii infection. We highlight the consequences of chronic infection for tissue-specific pathology and identify open questions in this area of host-Toxoplasma interactions.
Conflict of interest statement
Figures

References
-
- Speer CA, Dubey JP. Ultrastructure of early stages of infections in mice fed Toxoplasma gondii oocysts. Parasitology. 1998;116(pt 1):35–42. - PubMed

