Lamotrigine add-on therapy for drug-resistant generalised tonic-clonic seizures
- PMID: 32609387
- PMCID: PMC7387132
- DOI: 10.1002/14651858.CD007783.pub3
Lamotrigine add-on therapy for drug-resistant generalised tonic-clonic seizures
Abstract
Background: This is an update of the Cochrane Review first published in 2010; it includes one additional study. Primary generalised tonic-clonic seizures are a type of generalised seizure. Other types of seizures include: absence, myoclonic, and atonic seizures. Effective control of tonic-clonic seizures reduces the risk of injury and death, and improves quality of life. While most people achieve seizure control with one antiepileptic drug, around 30% do not, and require a combination of antiepileptic drugs.
Objectives: To assess the effectiveness and tolerability of add-on lamotrigine for drug-resistant primary generalised tonic-clonic seizures.
Search methods: For the latest update, we searched these databases on 19 March 2019: Cochrane Register of Studies (CRS) Web, MEDLINE Ovid, and the WHO International Clinical Trials Registry Platform (ICTRP). The CRS includes records from the Cochrane Epilepsy Group Specialized Register, CENTRAL, Embase, and ClinicalTrials.gov. We imposed no language restrictions. We also contacted GlaxoSmithKline, manufacturers of lamotrigine.
Selection criteria: Randomised controlled parallel or cross-over trials of add-on lamotrigine for people of any age with drug-resistant primary generalised tonic-clonic seizures.
Data collection and analysis: We followed standard Cochrane methodology; two review authors independently assessed trials for inclusion, evaluated risk of bias, extracted relevant data, and GRADE-assessed evidence. We investigated these outcomes: (1) 50% or greater reduction in primary generalised tonic-clonic seizure frequency; (2) seizure freedom; (3) treatment withdrawal; (4) adverse effects; (5) cognitive effects; and (6) quality of life. We used an intention-to-treat (ITT) population for all analyses, and presented results as risk ratios (RRs) with 95% confidence intervals (CIs); for adverse effects, we used 99% CIs to compensate for multiple hypothesis testing.
Main results: We included three studies (total 300 participants): two parallel-group studies and one cross-over study. We assessed varied risks of bias across studies; most limitations arose from the poor reporting of methodological details. We meta-analysed data extracted from the two parallel-group studies, and conducted a narrative synthesis for data from the cross-over study. Both parallel-group studies (270 participants) reported all dichotomous outcomes. Participants taking lamotrigine were almost twice as likely to attain a 50% or greater reduction in primary generalised tonic-clonic seizure frequency than those taking a placebo (RR 1.88, 95% CI 1.43 to 2.45; low-certainty evidence). The results between groups were inconclusive for the likelihood of seizure freedom (RR 1.55, 95% CI 0.89 to 2.72; very low-certainty evidence); treatment withdrawal (RR 1.20, 95% CI 0.72 to 1.99; very low-certainty evidence); and individual adverse effects: ataxia (RR 3.05, 99% CI 0.05 to 199.36); dizziness (RR 0.91, 99% CI 0.29 to 2.86; very low-certainty evidence); fatigue (RR 1.02, 99% CI 0.13 to 8.14; very low-certainty evidence); nausea (RR 1.60, 99% CI 0.48 to 5.32; very low-certainty evidence); and somnolence (RR 3.73, 99% CI 0.36 to 38.90; low-certainty evidence). The cross-over trial (26 participants) reported that 7/14 participants with generalised tonic-clonic seizures experienced a 50% or greater reduction in seizure frequency with add-on lamotrigine compared to placebo. The authors reported four treatment withdrawals, but did not specify during which treatment allocation they occurred. Rash (seven lamotrigine participants; zero placebo participants) and fatigue (five lamotrigine participants; zero placebo participants) were the most frequently reported adverse effects. None of the included studies measured cognition. One parallel-group study (N = 153) evaluated quality of life. They reported inconclusive results for the overall quality of life score between groups (P = 0.74).
Authors' conclusions: This review provides insufficient information to inform clinical practice. Low-certainty evidence suggests that lamotrigine reduces the rate of generalised tonic-clonic seizures by 50% or more. Very low-certainty evidence found inconclusive results between groups for all other outcomes. Therefore, we are uncertain to very uncertain that the results reported are accurate, and suggest that the true effect could be grossly different. More trials, recruiting larger populations, over longer periods, are necessary to determine lamotrigine's clinical use.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Rebecca Bresnahan: None to declare.
Mariangela Panebiano: None to declare.
Anthony G Marson:a consortium of pharmaceutical companies (GSK, EISAI, UCB Pharma) funded the National Audit of Seizure Management in Hospitals (NASH) through grants paid to the University of Liverpool. Professor Tony Marson is part funded by National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care North West Coast (NIHR CLAHRC NWC).
Figures
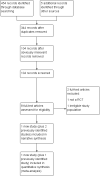





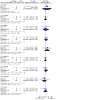
Update of
-
Lamotrigine adjunctive therapy for refractory generalized tonic-clonic seizures.Cochrane Database Syst Rev. 2010 Dec 8;(12):CD007783. doi: 10.1002/14651858.CD007783.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2020 Jul 1;7:CD007783. doi: 10.1002/14651858.CD007783.pub3. PMID: 21154386 Updated.
Similar articles
-
Lamotrigine add-on therapy for drug-resistant focal epilepsy.Cochrane Database Syst Rev. 2020 Mar 20;3(3):CD001909. doi: 10.1002/14651858.CD001909.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2023 Dec 11;12:CD001909. doi: 10.1002/14651858.CD001909.pub4. PMID: 32196639 Free PMC article. Updated.
-
Pregabalin add-on for drug-resistant focal epilepsy.Cochrane Database Syst Rev. 2019 Jul 9;7(7):CD005612. doi: 10.1002/14651858.CD005612.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2022 Mar 29;3:CD005612. doi: 10.1002/14651858.CD005612.pub5. PMID: 31287157 Free PMC article. Updated.
-
Lamotrigine adjunctive therapy for refractory generalized tonic-clonic seizures.Cochrane Database Syst Rev. 2010 Dec 8;(12):CD007783. doi: 10.1002/14651858.CD007783.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2020 Jul 1;7:CD007783. doi: 10.1002/14651858.CD007783.pub3. PMID: 21154386 Updated.
-
Brivaracetam add-on therapy for drug-resistant epilepsy.Cochrane Database Syst Rev. 2019 Mar 28;3(3):CD011501. doi: 10.1002/14651858.CD011501.pub2. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2022 Mar 14;3:CD011501. doi: 10.1002/14651858.CD011501.pub3. PMID: 30920649 Free PMC article. Updated.
-
Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents.Cochrane Database Syst Rev. 2021 Jan 21;1(1):CD003032. doi: 10.1002/14651858.CD003032.pub5. Cochrane Database Syst Rev. 2021. PMID: 33475151 Free PMC article.
Cited by
-
Case report: Sex-specific characteristics of epilepsy phenotypes associated with Xp22.31 deletion: a case report and review.Front Genet. 2023 Jun 6;14:1025390. doi: 10.3389/fgene.2023.1025390. eCollection 2023. Front Genet. 2023. PMID: 37347056 Free PMC article.
-
The Therapeutic Role of Lamotrigine in Borderline Personality Disorder: A Comprehensive Review of Outcomes, Mechanisms, and Treatment Strategies.Cureus. 2024 Aug 21;16(8):e67362. doi: 10.7759/cureus.67362. eCollection 2024 Aug. Cureus. 2024. PMID: 39310624 Free PMC article. Review.
-
Effect of Lamotrigine on Refractory Epilepsy: Clinical Outcomes and EEG Changes.Int J Gen Med. 2025 Jan 21;18:281-290. doi: 10.2147/IJGM.S505040. eCollection 2025. Int J Gen Med. 2025. PMID: 39867247 Free PMC article.
-
Psychobehavioural and Cognitive Adverse Events of Anti-Seizure Medications for the Treatment of Developmental and Epileptic Encephalopathies.CNS Drugs. 2022 Oct;36(10):1079-1111. doi: 10.1007/s40263-022-00955-9. Epub 2022 Oct 4. CNS Drugs. 2022. PMID: 36194365 Free PMC article. Review.
-
Lamotrigine add-on therapy for drug-resistant focal epilepsy.Cochrane Database Syst Rev. 2023 Dec 11;12(12):CD001909. doi: 10.1002/14651858.CD001909.pub4. Cochrane Database Syst Rev. 2023. PMID: 38078494 Free PMC article.
References
References to studies included in this review
Beran 1998 {published data only}
-
- Beran RG, Berkovic SF, Dunagan FM, Vajda FJ, Danta G, Black AB, et al. Double-blind placebo-controlled, crossover study of lamotrigine in treatment-resistant generalised epilepsy. Epilepsia 1998;39(12):1329-33. - PubMed
Biton 2005 {published data only}
-
- Biton V, Sackellares JC, Vuong A, Hammer AE, Barrett PS, Messenheimer JA. Double-blind, placebo-controlled study of lamotrigine in primary generalized tonic-clonic seizures. Neurology 2005;65:1737-43. - PubMed
-
- LAM40097. A Multi-Center, Double-Blind, Randomized, Placebo-Controlled, Parallel-Group Evaluation of Lamotrigine Adjunctive Therapy in Subjects With Primary Generalized Tonic-Clonic Seizures (Study GI267119). www.gsk-studyregister.com/study?uniqueStudyId=LAM40097 (accessed 20 May 2019).
-
- NCT00043901. Treatment of primary generalized tonic-clonic seizures with an investigational new drug [A multi-center, double-blind, randomized, placebo-controlled, parallel-group evaluation of lamotrigine adjunctive therapy in subjects with primary generalized tonic-clonic seizures]. https://clinicaltrials.gov/ct2/show/NCT00043901 (first received 16 August 2002).
-
- Tabbaa M, Kerls SP, Hammer AE, Vuong A, Messenheimer JA. Evaluation of lamotrigine adjunctive therapy for primary generalized tonic-clonic seizures in patients with a history of absence epilepsy. Epilepsia 2006;47(S4):47.
-
- Trevathan E, Kerls SP, Hammer AE, Vuong A, Messenheimer JA. Lamotrigine adjunctive therapy among children and adolescents with primary generalized tonic-clonic seizures. Paediatrics 2006;118(2):371-8. - PubMed
Biton 2010 {published data only}
-
- Biton V, Di Memmo J, Shukla R, Lee YY, Poverennova I, Demchenko V, et al. Adjunctive lamotrigine XR for primary generalized tonic-clonic seizures in a randomized, placebo-controlled study. Epilepsy & Behavior 2010;19(3):352-8. - PubMed
-
- EUCTR2004-004343-21. A multicenter, double-blind, randomized, parallel-group evaluation of LAMICTAL extended-release adjunctive therapy in subjects with primary generalized tonic-clonic seizures. www.clinicaltrialsregister.eu/ctr-search/search?query=2004-004343-21 (first received 12 January 2005).
-
- LAM100036. A multicenter, double-blind, randomized, parallel-group evaluation of LAMICTAL extended-release adjunctive therapy in subjects with primary generalized tonic-clonic seizures. www.gsk-studyregister.com/study?uniqueStudyId=LAM100036 (accessed 30 April 2019).
-
- NCT00104416. A multicenter, double-blind, randomized, parallel-group evaluation of LAMICTAL extended-release adjunctive therapy in patients with primary generalized tonic-clonic seizures. clinicaltrials.gov/ct2/show/study/NCT00104416 (first received 18 May 2010).
References to studies excluded from this review
Brzakovic 2012 {published data only}
-
- Brzakovic BB, Vezmar Kovacevic SD, Vucicevic KM, Miljkovic BR, Martinovic ZJ, Pokrajac MV, et al. Impact of age, weight and concomitant treatment on lamotrigine pharmacokinetics. Journal of Clinical Pharmacy and Therapeutics 2012;37(6):693-7. - PubMed
Chung 2009 {published data only}
Eriksson 1998 {published data only}
-
- Eriksson AS, Nergardh A, Hoppu K. The efficacy of lamotrigine in children and adolescents with refractory generalized epilepsy: a randomized, double-blind, crossover study. Epilepsia 1998;39(5):495-501. - PubMed
Motte 1997 {published data only}
-
- Motte J, Trevathan E, Arvidsson JFV, Barrera MN, Mullens EL, Manasco P. Lamotrigine for generalized seizures associated with the Lennox-Gastaut syndrome. New England Journal of Medicine 1997;337:1807-12. - PubMed
Sander 1990 {published data only}
-
- Sander JWA, Patsalos PN, Oxley JR, Hamilton MJ, Yuen WC. A randomised double-blind placebo-controlled add-on trial of lamotrigine in patients with severe epilepsy. Epilepsy Research 1990;6:221-6. - PubMed
Additional references
Bancaud 1989
-
- Bancaud J, Henriksen O, Rubio‐Donnadieu F, Seino M, Dreifuss FE, Kiffin Penry JK, International League Against Epilepsy. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia 1989;30:389-99. - PubMed
Besag 1998
-
- Besag FM, Berry DJ, Pool F, Newbery JE, Subel B. Carbamazepine toxicity with lamotrigine: pharmacokinetic or pharmacodynamic interaction? Epilepsia 1998;39(2):183-7. [PMID: ] - PubMed
Bloom 2017
Brigo 2019
Cockerell 1995
-
- Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the National General Practice Study of Epilepsy. Lancet 1995;346(8968):140-4. - PubMed
Deng 2013
-
- Deng Y, Wang M, Jiang L, Ma C, Xi Z, Li X, et al. A comparison of extracellular excitatory amino acids release inhibition of acute lamotrigine and topiramate treatment in the hippocampus of PTZ-kindled epileptic rats. Journal of Biomedical Nanotechnology 2013;9(6):1123-8. [PMID: ] - PubMed
Fiest 2017
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 9 May 2019. Hamilton (ON): McMaster University (developed by Evidence Prime).Available at gradepro.org.
Hajian‐Tillaki 2011
Hancock 2013
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
-
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports or randomized clinical trials: is blinding necessary? Controlled Clinical Trials 1996;17(1):1-12. - PubMed
Ketter 2005
-
- Ketter TA, Wang PW, Chandler RA, Alarcon AM, Becker OV, Nowakowska C, et al. Dermatology precautions and slower titration yield low incidence of lamotrigine treatment-emergent rash. The Journal of Clinical Psychiatry 2005;66(5):642-5. [PMID: ] - PubMed
Kumar 2018
Kwan 2010
-
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51(6):1069-77. [PMID: ] - PubMed
Leach 1995
-
- Leach LJ, Lees G, Riddall DR. Lamotrigine. In: Levy RH, editors(s). Mechanisms of Action in Antiepileptic Drugs. 4th edition. New York: Raven Press, 1995.
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lorberg 2009
-
- Lorberg B, Youssef NA, Bhagwagar Z. Lamotrigine-associated rash: to rechallenge or not to rechallenge? The International Journal of Neuropsychopharmacology 2009;12(2):257-65. [PMID: ] - PubMed
Marson 2007
Ramaratnam 2016
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rowland 2006
-
- Rowland A, Elliot DJ, Williams JA, Mackenzie PI, Dickinson RG, Miners JO. In vitro characterization of lamotrigine N2-glucuronidation and the lamotrigine-valproic acid interaction. Drug Metabolism and Disposition 2006;34(6):1055-62. [PMID: ] - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Weintraub 2005
-
- Weintraub D, Buchsbaum R, Resor SR Jr, Hirsch LJ. Effect of antiepileptic drug co-medication on lamotrigine clearance. Archives of Neurology 2005;62(9):1432-6. - PubMed
WHO 2019
-
- World Health Organization (WHO). Epilepsy. www.who.int/news-room/fact-sheets/detail/epilepsy (accessed 17 May 2019).
References to other published versions of this review
Tija‐Leong 2009
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

