Synthesis of Anhydroryanodol
- PMID: 32609506
- PMCID: PMC7477650
- DOI: 10.1021/jacs.0c05766
Synthesis of Anhydroryanodol
Abstract
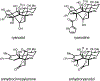
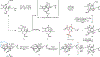
A stereoselective entry to ryanoids is described that culminates in the synthesis of anhydroryanodol and thus the formal total synthesis of ryanodol. The pathway described features an annulation reaction conceived to address the uniquely complex and highly oxygenated polycyclic skeleton common to members of this natural product class. It is demonstrated that metallacycle-mediated intramolecular coupling of an alkyne and a 1,3-diketone can proceed with a highly functionalized enyne and with outstanding levels of stereoselection. Furthermore, the first application of this technology in natural product synthesis is demonstrated here. More broadly, the advances described demonstrate the value that programs in natural product total synthesis have in advancing organic chemistry, here through the design and realization of an annulation reaction that accomplishes what previously established reactions do not.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
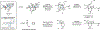
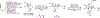

References
-
- Bélanger A; Berney DJF; Borschberg HJ; Brousseau R; Doutheau A; Durand R; Katayama H; Lapalme R; Leturc DM; Liao C-C; Maclachlan FN; Maffrand JP; Marazza F; Martino R; Moreau C; Saintlaurent L; Saintonge R; Soucy P; Ruest L; Deslongchamps P Total Synthesis of Ryanodol. Can. J. Chem 1979, 57, 3348–3354.
- Deslongchamps P; Bélanger A; Berney DJF; Borschberg HJ; Brousseau R; Doutheau A; Durand R; Katayama H; Lapalme R; Leturc DM; Liao C-C; Maclachlan FN; Maffrand JP; Marazza F; Martino R; Moreau C; Ruest L; Saintlaurent L; Saintonge R; Soucy P The Total Synthesis of (+)-Ryanodol. 1. General Strategy and Search for a Convenient Diene for the Construction of a Key Tricyclic Intermediate. Can. J. Chem 1990, 68, 115–126.
- Deslongchamps P; Bélanger A; Berney DJF; Borschberg HJ; Brousseau R; Doutheau A; Durand R; Katayama H; Lapalme R; Leturc DM; Liao C-C; Maclachlan FN; Maffrand JP; Marazza F; Martino R; Moreau C; Ruest L; Saintlaurent L; Saintonge R; Soucy P The Total Synthesis of (+)-Ryanodol. 2. Model Studies for Ring B and Ring C of (+)-Anhydroryanodol. Preparation of a Key Pentacyclic Intermediate. Can. J. Chem 1990, 68, 127–152.
- Deslongchamps P; Bélanger A; Berney DJF; Borschberg HJ; Brousseau R; Doutheau A; Durand R; Katayama H; Lapalme R; Leturc DM; Liao C-C; Maclachlan FN; Maffrand JP; Marazza F; Martino R; Moreau C; Ruest L; Saintlaurent L; Saintonge R; Soucy P The Total Synthesis of (+)-Ryanodol. 3. Preparation of (+)-Anhydroryanodol from a Key Pentacyclic Intermediate. Can. J. Chem 1990, 68, 153–185.
- Deslongchamps P; Bélanger A; Berney DJF; Borschberg HJ; Brousseau R; Doutheau A; Durand R; Katayama H; Lapalme R; Leturc DM; Liao C-C; Maclachlan FN; Maffrand JP; Marazza F; Martino R; Moreau C; Ruest L; Saintlaurent L; Saintonge R; Soucy P The Total Synthesis of (+)-Ryanodol. 4. Preparation of (+)-Ryanodol from (+)-Anhydroryanodol. Can. J. Chem 1990, 68, 186–192.
-
- Urabe D; Nagatomo M; Hagiwara K; Masuda K; Inoue M Symmetry-driven synthesis of 9-demethyl-10,15-dideoxyranodol. Chem. Sci 2013, 4, 1615–1619.
- Nagatomo M; Koshimizu M; Masuda K; Tabuchi T; Urabe D; Inoue M Total Synthesis of Ryanodol. J. Am. Chem. Soc 2014, 136, 5916–5919. - PubMed
- Nagatomo M; Hagiwara K; Masuda K; Koshimizu M; Kawamata T; Matsui Y; Urabe D; Inoue M Symmetry-Driven Strategy for the Assembly of the Core Tetracycle of (+)-Ryanodine: Synthetic Utility of a Cobalt-Catalyzed Olefin-Oxidation and a-Alkoxy Bridgehead Radical Reaction. Chem. Eur. J 2016, 22, 222–229. - PubMed
- Masuda K; Koshimizu M; Nagatomo M; Inoue M Asymmetric Total Synthesis of (+)-Ryanodol and (+)-Ryanodine. Chem. Eur. J 2016, 22, 230–236. - PubMed
-
- Chuang KV; Xu C; Reisman SEA 15-step synthesis of (+)-ryanodol. Science, 2016, 353, 912–915. - PMC - PubMed
- Xu C; Han A; Virgil SC; Reisman SE Chemical Synthesis of (+)-Ryanodine and (+)-20-Deoxyspiganthine. ACS Central Sci. 2017, 3, 278–282. - PMC - PubMed
- Han A; Tao Y; Reisman SEA 16-step synthesis of the isoryanodane diterpene (+)-perseanol. Nature, 2019, 573, 563–568. - PMC - PubMed
-
- Wood JL; Graeber JK; Njardarson JT Application of phenolic oxidation chemistry in synthesis: preparation of the BCE ring system of ryanodine. Tetrahedron 2003, 59, 8855–8858.
-
- Rogers EF; Koniuszy FR; Shavel J; Folkers K Plant Insecticides. 1. Ryanodine, a new alkaloid from Ryania speciose Vahl. J. Am. Chem. Soc 1948, 70, 3086–3088. - PubMed
- González-Coloma A; Cabrera R; Socorro Monzón AR; Fraga BM Persea Indica as a Natural Source of the Insecticide Ryanodol. Phytochemistry, 1993, 34, 397–400.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

