Influenza-induced thrombocytopenia is dependent on the subtype and sialoglycan receptor and increases with virus pathogenicity
- PMID: 32609845
- PMCID: PMC7362372
- DOI: 10.1182/bloodadvances.2020001640
Influenza-induced thrombocytopenia is dependent on the subtype and sialoglycan receptor and increases with virus pathogenicity
Abstract
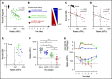
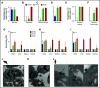
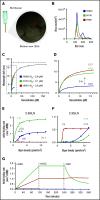
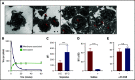
Thrombocytopenia is a common complication of influenza virus infection, and its severity predicts the clinical outcome of critically ill patients. The underlying cause(s) remain incompletely understood. In this study, in patients with an influenza A/H1N1 virus infection, viral load and platelet count correlated inversely during the acute infection phase. We confirmed this finding in a ferret model of influenza virus infection. In these animals, platelet count decreased with the degree of virus pathogenicity varying from 0% in animals infected with the influenza A/H3N2 virus, to 22% in those with the pandemic influenza A/H1N1 virus, up to 62% in animals with a highly pathogenic A/H5N1 virus infection. This thrombocytopenia is associated with virus-containing platelets that circulate in the blood. Uptake of influenza virus particles by platelets requires binding to sialoglycans and results in the removal of sialic acids by the virus neuraminidase, a trigger for hepatic clearance of platelets. We propose the clearance of influenza virus by platelets as a paradigm. These insights clarify the pathophysiology of influenza virus infection and show how severe respiratory infections, including COVID-19, may propagate thrombocytopenia and/or thromboembolic complications.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

Similar articles
-
H1N1, but not H3N2, influenza A virus infection protects ferrets from H5N1 encephalitis.J Virol. 2014 Mar;88(6):3077-91. doi: 10.1128/JVI.01840-13. Epub 2013 Dec 26. J Virol. 2014. PMID: 24371072 Free PMC article.
-
The influenza virus enigma.Cell. 2009 Feb 6;136(3):402-10. doi: 10.1016/j.cell.2009.01.029. Cell. 2009. PMID: 19203576 Free PMC article.
-
Tropism and infectivity of influenza virus, including highly pathogenic avian H5N1 virus, in ferret tracheal differentiated primary epithelial cell cultures.J Virol. 2013 Mar;87(5):2597-607. doi: 10.1128/JVI.02885-12. Epub 2012 Dec 19. J Virol. 2013. PMID: 23255802 Free PMC article.
-
Comparison of the pathology caused by H1N1, H5N1, and H3N2 influenza viruses.Arch Med Res. 2009 Nov;40(8):655-61. doi: 10.1016/j.arcmed.2009.10.001. Epub 2010 Jan 6. Arch Med Res. 2009. PMID: 20304252 Review.
-
Consequences of resistance: in vitro fitness, in vivo infectivity, and transmissibility of oseltamivir-resistant influenza A viruses.Influenza Other Respir Viruses. 2013 Jan;7 Suppl 1(Suppl 1):50-7. doi: 10.1111/irv.12044. Influenza Other Respir Viruses. 2013. PMID: 23279897 Free PMC article. Review.
Cited by
-
In Vivo Models to Study the Pathogenesis of Extra-Respiratory Complications of Influenza A Virus Infection.Viruses. 2021 May 6;13(5):848. doi: 10.3390/v13050848. Viruses. 2021. PMID: 34066589 Free PMC article. Review.
-
The role of platelets in central hubs of inflammation: A literature review.Medicine (Baltimore). 2024 May 10;103(19):e38115. doi: 10.1097/MD.0000000000038115. Medicine (Baltimore). 2024. PMID: 38728509 Free PMC article. Review.
-
Life-Threatening Anemia and Thrombocytopenia in a Toddler with Influenza B: Case Report and Literature Review.Children (Basel). 2025 May 14;12(5):632. doi: 10.3390/children12050632. Children (Basel). 2025. PMID: 40426810 Free PMC article.
-
Multivalent Affinity Profiling: Direct Visualization of the Superselective Binding of Influenza Viruses.ACS Nano. 2021 May 25;15(5):8525-8536. doi: 10.1021/acsnano.1c00166. Epub 2021 May 12. ACS Nano. 2021. PMID: 33978406 Free PMC article.
-
Asymptomatic thrombocytopenia after nintedanib initiation in a patient with progressive pulmonary fibrosis: a case report and review of literature.J Med Case Rep. 2024 Sep 29;18(1):451. doi: 10.1186/s13256-024-04790-y. J Med Case Rep. 2024. PMID: 39342394 Free PMC article. Review.
References
-
- George JN. Platelets. Lancet. 2000;355(9214):1531-1539. - PubMed
-
- Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93(1):327-358. - PubMed
-
- Estcourt LJ, Stanworth SJ, Doree C, Hopewell S, Trivella M, Murphy MF. Comparison of different platelet count thresholds to guide administration of prophylactic platelet transfusion for preventing bleeding in people with haematological disorders after myelosuppressive chemotherapy or stem cell transplantation. Cochrane Database Syst Rev. 2015;(11):CD010983. - PMC - PubMed
-
- Broadley SP, Plaumann A, Coletti R, et al. . Dual-track clearance of circulating bacteria balances rapid restoration of blood sterility with induction of adaptive immunity. Cell Host Microbe. 2016;20(1):36-48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

