Impact of COVID-19 on liver transplantation in Europe: alert from an early survey of European Liver and Intestine Transplantation Association and European Liver Transplant Registry
- PMID: 32609908
- PMCID: PMC7361228
- DOI: 10.1111/tri.13680
Impact of COVID-19 on liver transplantation in Europe: alert from an early survey of European Liver and Intestine Transplantation Association and European Liver Transplant Registry
Abstract
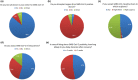
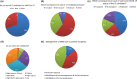
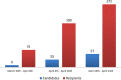
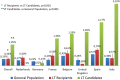
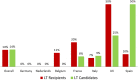
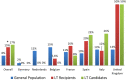
There are scarce data on the impact of COVID-19 pandemic on liver transplantation (LT) in Europe. The aim of this study was to obtain a preliminary data on incidence, management, and outcome of COVID-19 in liver transplant recipients and candidates in Europe. An Internet-based survey was sent to the centers affiliated with European Liver Transplant Registry (ELTR). One hundred nine out of 149 (73%) of ELTR centers located in 28 European countries (93%) responded. Ninety-four (86%) of the centers tested all donors, and 75 (69%) centers tested all LT recipients for SARS-CoV-2. Seventy-three (67%) centers selected recipients for LT in the COVID-19 pandemic, whereas 33% did not. Eighty-eight centers reported COVID-19 infection in 57 LT candidates and in 272 LT recipients. Overall crude incidence of COVID-19 among LT candidates and recipients was estimated 1.05% (range 0.5-20%) and 0.34% (range 0.1-4.8%), respectively, and it was significantly higher among candidates (P < 0.001). Crude rate of death was 18% (10/57) among candidates and 15% (36/244) among recipients. This first large-scale European snapshot study clearly shows that both LT candidates and recipients are at a high risk for COVID-19. These results plead for an early and pro-active screening of COVID-19 symptoms in these populations.
Keywords: COVID-19; SARS-CoV-2; liver recipient; liver transplantation; mortality; survey.
© 2020 Steunstichting ESOT. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures


Comment in
-
Increased incidence of COVID-19 among liver transplant patients in Europe.Transpl Int. 2020 Dec;33(12):1823-1824. doi: 10.1111/tri.13729. Epub 2020 Sep 20. Transpl Int. 2020. PMID: 32881089 No abstract available.
-
Coronavirus disease 2019 and transplantation: tackling the challenges of SARS-CoV-2 infection in waiting list candidates.Transpl Int. 2020 Dec;33(12):1830-1832. doi: 10.1111/tri.13742. Epub 2020 Sep 28. Transpl Int. 2020. PMID: 32936963 No abstract available.
-
Reply to Rodriguez-Peralvarez et al.Transpl Int. 2020 Dec;33(12):1825-1826. doi: 10.1111/tri.13765. Epub 2020 Nov 9. Transpl Int. 2020. PMID: 33037657 Free PMC article. No abstract available.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

