Rapid, Sensitive, Full-Genome Sequencing of Severe Acute Respiratory Syndrome Coronavirus 2
- PMID: 32610037
- PMCID: PMC7510745
- DOI: 10.3201/eid2610.201800
Rapid, Sensitive, Full-Genome Sequencing of Severe Acute Respiratory Syndrome Coronavirus 2
Abstract
We describe validated protocols for generating high-quality, full-length severe acute respiratory syndrome coronavirus 2 genomes from primary samples. One protocol uses multiplex reverse transcription PCR, followed by MinION or MiSeq sequencing; the other uses singleplex, nested reverse transcription PCR and Sanger sequencing. These protocols enable sensitive virus sequencing in different laboratory environments.
Keywords: COVID-19; SARS-CoV-2; coronavirus; coronavirus disease; genomics; high-throughput nucleotide sequencing; respiratory infections; severe acute respiratory syndrome coronavirus 2; viruses; whole-genome sequencing; zoonoses.
Figures
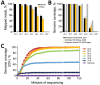
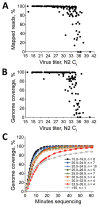
References
-
- Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al.; Washington State 2019-nCoV Case Investigation Team. Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–36. 10.1056/NEJMoa2001191 - DOI - PMC - PubMed
-
- Patel A, Jernigan DB, Abdirizak F, Abedi G, Aggarwal S, Albina D, et al.; 2019-nCoV CDC Response Team. 2019-nCoV CDC Response Team. Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak—United States, December 31, 2019–February 4, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:140–6. 10.15585/mmwr.mm6905e1 - DOI - PMC - PubMed
-
- World Health Organization. Coronavirus disease 2019. (COVID-19) situation report 141 [cited 2020 Jun 9]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

