Histone Acetyltransferase MOF Blocks Acquisition of Quiescence in Ground-State ESCs through Activating Fatty Acid Oxidation
- PMID: 32610040
- PMCID: PMC7758074
- DOI: 10.1016/j.stem.2020.06.005
Histone Acetyltransferase MOF Blocks Acquisition of Quiescence in Ground-State ESCs through Activating Fatty Acid Oxidation
Abstract
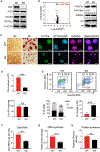
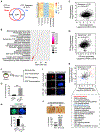
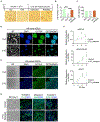
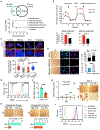
Self-renewing embryonic stem cells (ESCs) respond to environmental cues by exiting pluripotency or entering a quiescent state. The molecular basis underlying this fate choice remains unclear. Here, we show that histone acetyltransferase MOF plays a critical role in this process through directly activating fatty acid oxidation (FAO) in the ground-state ESCs. We further show that the ground-state ESCs particularly rely on elevated FAO for oxidative phosphorylation (OXPHOS) and energy production. Mof deletion or FAO inhibition induces bona fide quiescent ground-state ESCs with an intact core pluripotency network and transcriptome signatures akin to the diapaused epiblasts in vivo. Mechanistically, MOF/FAO inhibition acts through reducing mitochondrial respiration (i.e., OXPHOS), which in turn triggers reversible pluripotent quiescence specifically in the ground-state ESCs. The inhibition of FAO/OXPHOS also induces quiescence in naive human ESCs. Our study suggests a general function of the MOF/FAO/OXPHOS axis in regulating cell fate determination in stem cells.
Keywords: FAO; MOF; cell fate decision; embryo development; epigenetics; quiescence; self-renewal; stem cell metabolism.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests
Figures



Similar articles
-
Derivation of trophoblast stem cells from naïve human pluripotent stem cells.Elife. 2020 Feb 12;9:e52504. doi: 10.7554/eLife.52504. Elife. 2020. PMID: 32048992 Free PMC article.
-
Epigenetic modifiers promote mitochondrial biogenesis and oxidative metabolism leading to enhanced differentiation of neuroprogenitor cells.Cell Death Dis. 2018 Mar 2;9(3):360. doi: 10.1038/s41419-018-0396-1. Cell Death Dis. 2018. PMID: 29500414 Free PMC article.
-
Fatty Acid Oxidation Fuels Mitochondrial Respiration to Drive Epidermal Stem Cell Fate and Barrier Regeneration.Res Sq [Preprint]. 2025 Jun 4:rs.3.rs-6636875. doi: 10.21203/rs.3.rs-6636875/v1. Res Sq. 2025. PMID: 40502760 Free PMC article. Preprint.
-
Moving toward totipotency: the molecular and cellular features of totipotent and naive pluripotent stem cells.Hum Reprod Update. 2025 Jul 1;31(4):361-391. doi: 10.1093/humupd/dmaf006. Hum Reprod Update. 2025. PMID: 40299455 Review.
-
Pluripotency genes of mammals: a network at work.Front Bioeng Biotechnol. 2025 Jun 12;13:1578499. doi: 10.3389/fbioe.2025.1578499. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40574823 Free PMC article. Review.
Cited by
-
ScatLay: utilizing transcriptome-wide noise for identifying and visualizing differentially expressed genes.Sci Rep. 2020 Oct 15;10(1):17483. doi: 10.1038/s41598-020-74564-1. Sci Rep. 2020. PMID: 33060728 Free PMC article.
-
In Vitro Derivation of Quiescent Mouse Embryonic Stem Cells Based on Distinct Mitochondrial Activity.STAR Protoc. 2020 Oct 20;1(3):100136. doi: 10.1016/j.xpro.2020.100136. eCollection 2020 Dec 18. STAR Protoc. 2020. PMID: 33377030 Free PMC article.
-
Metabolic regulation of the hallmarks of stem cell biology.Cell Stem Cell. 2024 Feb 1;31(2):161-180. doi: 10.1016/j.stem.2024.01.003. Cell Stem Cell. 2024. PMID: 38306993 Free PMC article. Review.
-
Glucocorticoid receptor modulates myeloid-derived suppressor cell function via mitochondrial metabolism in immune thrombocytopenia.Cell Mol Immunol. 2022 Jul;19(7):764-776. doi: 10.1038/s41423-022-00859-0. Epub 2022 Apr 12. Cell Mol Immunol. 2022. PMID: 35414712 Free PMC article.
-
FOXO1-mediated lipid metabolism maintains mammalian embryos in dormancy.Nat Cell Biol. 2024 Feb;26(2):181-193. doi: 10.1038/s41556-023-01325-3. Epub 2024 Jan 4. Nat Cell Biol. 2024. PMID: 38177284 Free PMC article.
References
-
- Atlasi Y, Megchelenbrink W, Peng T, Habibi E, Joshi O, Wang SY, Wang C, Logie C, Poser I, Marks H, and Stunnenberg HG (2019). Epigenetic modulation of a hardwired 3D chromatin landscape in two naive states of pluripotency. Nat. Cell Biol 21, 568–578. - PubMed
-
- Barbehenn EK, Wales RG, and Lowry OH (1978). Measurement of metabolites in single preimplantation embryos; a new means to study metabolic control in early embryos. J. Embryol. Exp. Morphol 43, 29–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

