Parallel Glyco-SPOT Synthesis of Glycopeptide Libraries
- PMID: 32610041
- PMCID: PMC7556346
- DOI: 10.1016/j.chembiol.2020.06.007
Parallel Glyco-SPOT Synthesis of Glycopeptide Libraries
Abstract
Glycan recognition is typically studied using free glycans, but glycopeptide presentations represent more physiological conditions for glycoproteins. To facilitate studies of glycopeptide recognition, we developed Glyco-SPOT synthesis, which enables the parallel production of diverse glycopeptide libraries at microgram scales. The method uses a closed system for prolonged reactions required for coupling Fmoc-protected glycoamino acids, including O-, N-, and S-linked glycosides, and release conditions to prevent side reactions. To optimize reaction conditions and sample reaction progress, we devised a biopsy testing method. We demonstrate the efficient utilization of such microscale glycopeptide libraries to determine the specificity of glycan-recognizing antibodies (e.g., CTD110.6) using microarrays, enzyme specificity on-array and in-solution (e.g., ST6GalNAc1, GCNT1, and T-synthase), and binding kinetics using fluorescence polarization. We demonstrated that the glycosylation on these peptides can be expanded using glycosyltransferases both in-solution and on-array. This technology will promote the discovery of biological functions of peptide modifications by glycans.
Keywords: CTD110.6; GCNT1; S-GlcNAcylation; ST6GalNAc1; TSynthase; glycan; glycopeptide; glycopeptide library; glycosyltransferase; mass spectrometry; microarray.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no conflicts of interest.
Figures


 indicates 5(6)-carboxyfluorescein on the N-terminus. For complete list of sequences see also Tables S1 and S2.
indicates 5(6)-carboxyfluorescein on the N-terminus. For complete list of sequences see also Tables S1 and S2.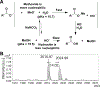




References
-
- Apweiler R, Hermjakob H, and Sharon N (1999). On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta 1473, 4–8. - PubMed
-
- Bora U (2011). An Eco-friendly and Mild Process for Deacetylation Reactions in Water. Asian J Chem 23, 941–942.
-
- Bubb WA (2003). NMR spectroscopy in the study of carbohydrates: Characterizing the structural complexity. Concepts in Magnetic Resonance Part A 19A, 1–19.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

