The Lysosome at the Intersection of Cellular Growth and Destruction
- PMID: 32610045
- PMCID: PMC7959181
- DOI: 10.1016/j.devcel.2020.06.010
The Lysosome at the Intersection of Cellular Growth and Destruction
Abstract
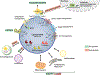
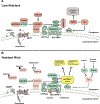
The lysosome is an essential catabolic organelle that consumes cellular biomass to regenerate basic building blocks that can fuel anabolic reactions. This simple view has evolved more recently to integrate novel functions of the lysosome as a key signaling center, which can steer the metabolic trajectory of cells in response to changes in nutrients, growth factors, and stress. Master protein kinases and transcription factors mediate the growth-promoting and catabolic activities of the lysosome and undergo a complex interplay that enables cellular adaptation to ever-changing metabolic conditions. Understanding how this coordination occurs will shed light on the fundamental logic of how the lysosome functions to control growth in the context of development, tissue homeostasis, and cancer.
Keywords: TFEB; anabolism; autophagy; catabolism; lysosome; mTORC1; nutrient sensing.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests R.Z. is a co-founder, shareholder, and consultant for Frontier Medicines Corp.
Figures
References
-
- Baldassari S, Picard F, Verbeek NE, van Kempen M, Brilstra EH, Lesca G, Conti V, Guerrini R, Bisulli F, Licchetta L, Pippucci T, Tinuper P, Hirsch E, de Saint Martin A, Chelly J, Rudolf G, Chipaux M, Ferrand-Sorbets S, Dorfmüller G, Sisodiya S, Balestrini S, Schoeler N, Hernandez-Hernandez L, Krithika S, Oegema R, Hagebeuk E, Gunning B, Deckers C, Berghuis B, Wegner I, Niks E, Jansen FE, Braun K, de Jong D, Rubboli G, Talvik I, Sander V, Uldall P, Jacquemont M-L, Nava C, Leguern E, Julia S, Gambardella A, d’Orsi G, Crichiutti G, Faivre L, Darmency V, Benova B, Krsek P, Biraben A, Lebre A-S, Jennesson M, Sattar S, Marchal C, Nordli DR, Lindstrom K, Striano P, Lomax LB, Kiss C, Bartolomei F, Lepine AF, Schoonjans A-S, Stouffs K, Jansen A, Panagiotakaki E, Ricard-Mousnier B, Thevenon J, de Bellescize J, Catenoix H, Dorn T, Zenker M, Müller-Schlüter K, Brandt C, Krey I, Polster T, Wolff M, Balci M, Rostasy K, Achaz G, Zacher P, Becher T, Cloppenborg T, Yuskaitis CJ, Weckhuysen S, Poduri A, Lemke JR, Møller RS, Baulac S, 2019. The landscape of epilepsy-related GATOR1 variants. Genet. Med. Off. J. Am. Coll. Med. Genet 21, 398–408. - PMC - PubMed

