Genetic loss of AMPK-glycogen binding destabilises AMPK and disrupts metabolism
- PMID: 32610071
- PMCID: PMC7393401
- DOI: 10.1016/j.molmet.2020.101048
Genetic loss of AMPK-glycogen binding destabilises AMPK and disrupts metabolism
Abstract
Objective: Glycogen is a major energy reserve in liver and skeletal muscle. The master metabolic regulator AMP-activated protein kinase (AMPK) associates with glycogen via its regulatory β subunit carbohydrate-binding module (CBM). However, the physiological role of AMPK-glycogen binding in energy homeostasis has not been investigated in vivo. This study aimed to determine the physiological consequences of disrupting AMPK-glycogen interactions.
Methods: Glycogen binding was disrupted in mice via whole-body knock-in (KI) mutation of either the AMPK β1 (W100A) or β2 (W98A) isoform CBM. Systematic whole-body, tissue and molecular phenotyping was performed in KI and respective wild-type (WT) mice.
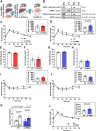
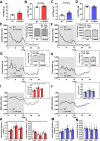
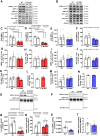
Results: While β1 W100A KI did not affect whole-body metabolism or exercise capacity, β2 W98A KI mice displayed increased adiposity and impairments in whole-body glucose handling and maximal exercise capacity relative to WT. These KI mutations resulted in reduced total AMPK protein and kinase activity in liver and skeletal muscle of β1 W100A and β2 W98A, respectively, versus WT mice. β1 W100A mice also displayed loss of fasting-induced liver AMPK total and α-specific kinase activation relative to WT. Destabilisation of AMPK was associated with increased fat deposition in β1 W100A liver and β2 W98A skeletal muscle versus WT.
Conclusions: These results demonstrate that glycogen binding plays critical roles in stabilising AMPK and maintaining cellular, tissue and whole-body energy homeostasis.
Keywords: AMP-activated protein kinase; Carbohydrate-binding module; Cellular energy sensing; Exercise; Liver; Skeletal muscle.
Copyright © 2020 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures

References
-
- Xiao B., Heath R., Saiu P., Leiper F.C., Leone P., Jing C. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449(7161):496–500. - PubMed
-
- Townley R., Shapiro L. Crystal structures of the adenylate sensor from fission yeast AMP-activated protein kinase. Science. 2007;315(5819):1726–1729. - PubMed
-
- Polekhina G., Gupta A., Michell B.J., van Denderen B., Murthy S., Feil S.C. AMPK beta subunit targets metabolic stress sensing to glycogen. Current Biology. 2003;13(10):867–871. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

