Origin and Function of Stress-Induced IL-6 in Murine Models
- PMID: 32610084
- PMCID: PMC7384974
- DOI: 10.1016/j.cell.2020.05.054
Origin and Function of Stress-Induced IL-6 in Murine Models
Erratum in
-
Origin and Function of Stress-Induced IL-6 in Murine Models.Cell. 2020 Sep 17;182(6):1660. doi: 10.1016/j.cell.2020.08.044. Cell. 2020. PMID: 32946784 No abstract available.
Abstract
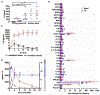
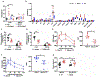
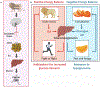
Acute psychological stress has long been known to decrease host fitness to inflammation in a wide variety of diseases, but how this occurs is incompletely understood. Using mouse models, we show that interleukin-6 (IL-6) is the dominant cytokine inducible upon acute stress alone. Stress-inducible IL-6 is produced from brown adipocytes in a beta-3-adrenergic-receptor-dependent fashion. During stress, endocrine IL-6 is the required instructive signal for mediating hyperglycemia through hepatic gluconeogenesis, which is necessary for anticipating and fueling "fight or flight" responses. This adaptation comes at the cost of enhancing mortality to a subsequent inflammatory challenge. These findings provide a mechanistic understanding of the ontogeny and adaptive purpose of IL-6 as a bona fide stress hormone coordinating systemic immunometabolic reprogramming. This brain-brown fat-liver axis might provide new insights into brown adipose tissue as a stress-responsive endocrine organ and mechanistic insight into targeting this axis in the treatment of inflammatory and neuropsychiatric diseases.
Keywords: IL-6; acute stress; beta-adrenergic receptors; brown adipose tissue; gluconeogenesis; immunometabolism; inflammation; neuroendocrine-immune axis; neuroimmunology; tolerance.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures




Comment in
-
The Link between Stress and IL-6 Is Heating Up.Cell Metab. 2020 Aug 4;32(2):152-153. doi: 10.1016/j.cmet.2020.07.011. Cell Metab. 2020. PMID: 32755607 Free PMC article.
References
-
- Abreu-Vieira G, Hagberg CE, Spalding KL, Cannon B, and Nedergaard J (2015). Adrenergically stimulated blood flow in brown adipose tissue is not dependent on thermogenesis. American journal of physiology Endocrinology and metabolism 308, E822–829. - PubMed
-
- Banzet S, Koulmann N, Simler N, Sanchez H, Chapot R, Serrurier B, Peinnequin A, and Bigard X (2009). Control of gluconeogenic genes during intense/prolonged exercise: hormone-independent effect of muscle-derived IL-6 on hepatic tissue and PEPCK mRNA. J Appl Physiol (1985) 107, 1830–1839. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

