Late Embryogenesis Abundant Protein-Client Protein Interactions
- PMID: 32610443
- PMCID: PMC7412488
- DOI: 10.3390/plants9070814
Late Embryogenesis Abundant Protein-Client Protein Interactions
Abstract
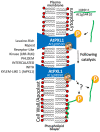
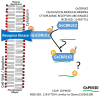
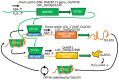
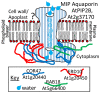
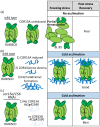
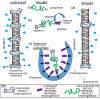
The intrinsically disordered proteins belonging to the LATE EMBRYOGENESIS ABUNDANT protein (LEAP) family have been ascribed a protective function over an array of intracellular components. We focus on how LEAPs may protect a stress-susceptible proteome. These examples include instances of LEAPs providing a shield molecule function, possibly by instigating liquid-liquid phase separations. Some LEAPs bind directly to their client proteins, exerting a holdase-type chaperonin function. Finally, instances of LEAP-client protein interactions have been documented, where the LEAP modulates (interferes with) the function of the client protein, acting as a surreptitious rheostat of cellular homeostasis. From the examples identified to date, it is apparent that client protein modulation also serves to mitigate stress. While some LEAPs can physically bind and protect client proteins, some apparently bind to assist the degradation of the client proteins with which they associate. Documented instances of LEAP-client protein binding, even in the absence of stress, brings to the fore the necessity of identifying how the LEAPs are degraded post-stress to render them innocuous, a first step in understanding how the cell regulates their abundance.
Keywords: desiccation; late embryogenesis abundant; natural protection and repair mechanism; protein interaction; seed; stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Gusev O., Suetsugu Y., Cornette R., Kawashima T., Logacheva M.D., Kondrashov A.S., Penin A.A., Hatanaka R., Kikuta S., Shimura S., et al. Comparative genome sequencing reveals genomic signature of extreme desiccation tolerance in the anhydrobiotic midge. Nat. Commun. 2014;5:4784. doi: 10.1038/ncomms5784. - DOI - PMC - PubMed
Publication types
Grants and funding
- Hatch Project 1019088/Agricultural Research Service
- W911NF-19-1-0222/Army Research Office
- six-month sabbatical for (IA)/Higher Education Commission, Pakistan
- PhD student scholarship ICSN/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
- PhD student scholarship FECBP/Sebastiao Maia de Andrade Foundation
LinkOut - more resources
Full Text Sources

