Low Vitamin B12 and Lipid Metabolism: Evidence from Pre-Clinical and Clinical Studies
- PMID: 32610503
- PMCID: PMC7400011
- DOI: 10.3390/nu12071925
Low Vitamin B12 and Lipid Metabolism: Evidence from Pre-Clinical and Clinical Studies
Abstract
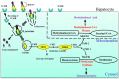
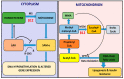
Obesity is a worldwide epidemic responsible for 5% of global mortality. The risks of developing other key metabolic disorders like diabetes, hypertension and cardiovascular diseases (CVDs) are increased by obesity, causing a great public health concern. A series of epidemiological studies and animal models have demonstrated a relationship between the importance of vitamin B12 (B12) and various components of metabolic syndrome. High prevalence of low B12 levels has been shown in European (27%) and South Indian (32%) patients with type 2 diabetes (T2D). A longitudinal prospective study in pregnant women has shown that low B12 status could independently predict the development of T2D five years after delivery. Likewise, children born to mothers with low B12 levels may have excess fat accumulation which in turn can result in higher insulin resistance and risk of T2D and/or CVD in adulthood. However, the independent role of B12 on lipid metabolism, a key risk factor for cardiometabolic disorders, has not been explored to a larger extent. In this review, we provide evidence from pre-clinical and clinical studies on the role of low B12 status on lipid metabolism and insights on the possible epigenetic mechanisms including DNA methylation, micro-RNA and histone modifications. Although, there are only a few association studies of B12 on epigenetic mechanisms, novel approaches to understand the functional changes caused by these epigenetic markers are warranted.
Keywords: cardiovascular disease (CVD); lipid metabolism; metabolic syndrome (MetS); obesity; type 2 diabetes mellitus (T2D); vitamin B12 (B12).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

