Multiple Herpes Simplex Virus-1 (HSV-1) Reactivations Induce Protein Oxidative Damage in Mouse Brain: Novel Mechanisms for Alzheimer's Disease Progression
- PMID: 32610629
- PMCID: PMC7409037
- DOI: 10.3390/microorganisms8070972
Multiple Herpes Simplex Virus-1 (HSV-1) Reactivations Induce Protein Oxidative Damage in Mouse Brain: Novel Mechanisms for Alzheimer's Disease Progression
Abstract
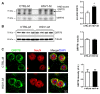
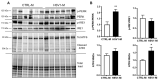
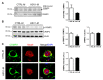
Compelling evidence supports the role of oxidative stress in Alzheimer's disease (AD) pathophysiology. Interestingly, Herpes simplex virus-1 (HSV-1), a neurotropic virus that establishes a lifelong latent infection in the trigeminal ganglion followed by periodic reactivations, has been reportedly linked both to AD and to oxidative stress conditions. Herein, we analyzed, through biochemical and redox proteomic approaches, the mouse model of recurrent HSV-1 infection we previously set up, to investigate whether multiple virus reactivations induced oxidative stress in the mouse brain and affected protein function and related intracellular pathways. Following multiple HSV-1 reactivations, we found in mouse brains increased levels of oxidative stress hallmarks, including 4-hydroxynonenal (HNE), and 13 HNE-modified proteins whose levels were found significantly altered in the cortex of HSV-1-infected mice compared to controls. We focused on two proteins previously linked to AD pathogenesis, i.e., glucose-regulated protein 78 (GRP78) and collapsin response-mediated protein 2 (CRMP2), which are involved in the unfolded protein response (UPR) and in microtubule stabilization, respectively. We found that recurrent HSV-1 infection disables GRP78 function and activates the UPR, whereas it prevents CRMP2 function in mouse brains. Overall, these data suggest that repeated HSV-1 reactivation into the brain may contribute to neurodegeneration also through oxidative damage.
Keywords: Alzheimer’s disease; HSV-1; Herpes simplex virus-1; oxidative stress; redox proteomics.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

