Combinatory Treatment with Oseltamivir and Itraconazole Targeting Both Virus and Host Factors in Influenza A Virus Infection
- PMID: 32610711
- PMCID: PMC7412427
- DOI: 10.3390/v12070703
Combinatory Treatment with Oseltamivir and Itraconazole Targeting Both Virus and Host Factors in Influenza A Virus Infection
Abstract
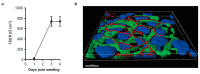
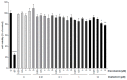
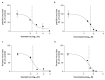
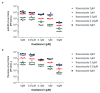
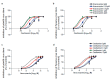
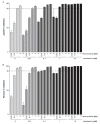
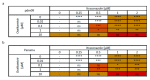
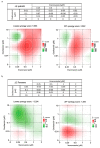
Influenza virus infections and their associated morbidity and mortality are a major threat to global health. Vaccination is an effective influenza prevention measure; however, the effectiveness is challenged by the rapid changes in the influenza virus genome leading to viral adaptation. Emerging viral resistance to the neuraminidase inhibitor oseltamivir limits the treatment of acute influenza infections. Targeting influenza virus‑host interactions is a new and emerging field, and therapies based on the combination of virus‑ and host‑directed drugs might significantly improve treatment success. We therefore assessed the combined treatment with oseltamivir and the repurposed antifungal drug itraconazole on infection of polarized broncho‑epithelial Calu-3 cells with pdm09 or Panama influenza A virus strains. We detected significantly stronger antiviral activities in the combined treatment compared to monotherapy with oseltamivir, permitting lower concentrations of the drug than required for the single treatments. Bliss independence drug interaction analysis indicated that both drugs acted independently of each other. The additional antiviral effect of itraconazole might safeguard patients infected with influenza virus strains with heightened oseltamivir resistance.
Keywords: additivity; combination therapy; drug repurposing; host‑directed therapy; influenza A virus; itraconazole; oseltamivir.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Repurposing of Drugs as Novel Influenza Inhibitors From Clinical Gene Expression Infection Signatures.Front Immunol. 2019 Jan 29;10:60. doi: 10.3389/fimmu.2019.00060. eCollection 2019. Front Immunol. 2019. PMID: 30761132 Free PMC article.
-
In vitro activity of favipiravir and neuraminidase inhibitor combinations against oseltamivir-sensitive and oseltamivir-resistant pandemic influenza A (H1N1) virus.Arch Virol. 2014 Jun;159(6):1279-91. doi: 10.1007/s00705-013-1922-1. Epub 2013 Dec 6. Arch Virol. 2014. PMID: 24311151
-
Effect of aloin on viral neuraminidase and hemagglutinin-specific T cell immunity in acute influenza.Phytomedicine. 2019 Nov;64:152904. doi: 10.1016/j.phymed.2019.152904. Epub 2019 Apr 4. Phytomedicine. 2019. PMID: 31454654
-
Influenza virus susceptibility and resistance to oseltamivir.Antivir Ther. 2007;12(4 Pt B):603-16. Antivir Ther. 2007. PMID: 17944268 Review.
-
Oseltamivir: a review of its use in influenza.Drugs. 2001;61(2):263-83. doi: 10.2165/00003495-200161020-00011. Drugs. 2001. PMID: 11270942 Review.
Cited by
-
The MEK1/2 Inhibitor ATR-002 (Zapnometinib) Synergistically Potentiates the Antiviral Effect of Direct-Acting Anti-SARS-CoV-2 Drugs.Pharmaceutics. 2022 Aug 25;14(9):1776. doi: 10.3390/pharmaceutics14091776. Pharmaceutics. 2022. PMID: 36145524 Free PMC article.
-
The real-world safety of oseltamivir and baloxavir marboxil in children: a disproportionality analysis of the FDA adverse event reporting system.Front Pharmacol. 2024 Jul 10;15:1391003. doi: 10.3389/fphar.2024.1391003. eCollection 2024. Front Pharmacol. 2024. PMID: 39050747 Free PMC article.
-
Targeting the endolysosomal host-SARS-CoV-2 interface by clinically licensed functional inhibitors of acid sphingomyelinase (FIASMA) including the antidepressant fluoxetine.Emerg Microbes Infect. 2020 Dec;9(1):2245-2255. doi: 10.1080/22221751.2020.1829082. Emerg Microbes Infect. 2020. PMID: 32975484 Free PMC article.
-
Exploring the Protective Effect of Food Drugs against Viral Diseases: Interaction of Functional Food Ingredients and SARS-CoV-2, Influenza Virus, and HSV.Life (Basel). 2023 Feb 1;13(2):402. doi: 10.3390/life13020402. Life (Basel). 2023. PMID: 36836758 Free PMC article. Review.
-
Cholesterol and Cholesterol-Lowering Medications in COVID-19-An Unresolved Matter.Int J Mol Sci. 2024 Sep 29;25(19):10489. doi: 10.3390/ijms251910489. Int J Mol Sci. 2024. PMID: 39408818 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

