Gut Microbiota Restricts NETosis in Acute Mesenteric Ischemia-Reperfusion Injury
- PMID: 32611241
- PMCID: PMC7484055
- DOI: 10.1161/ATVBAHA.120.314491
Gut Microbiota Restricts NETosis in Acute Mesenteric Ischemia-Reperfusion Injury
Abstract
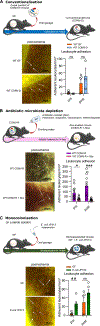
Objective: Recruitment of neutrophils and formation of neutrophil extracellular traps (NETs) contribute to lethality in acute mesenteric infarction. To study the impact of the gut microbiota in acute mesenteric infarction, we used gnotobiotic mouse models to investigate whether gut commensals prime the reactivity of neutrophils towards formation of neutrophil extracellular traps (NETosis). Approach and Results: We applied a mesenteric ischemia-reperfusion (I/R) injury model to germ-free (GF) and colonized C57BL/6J mice. By intravital imaging, we quantified leukocyte adherence and NET formation in I/R-injured mesenteric venules. Colonization with gut microbiota or monocolonization with Escherichia coli augmented the adhesion of leukocytes, which was dependent on the TLR4 (Toll-like receptor-4)/TRIF (TIR-domain-containing adapter-inducing interferon-β) pathway. Although neutrophil accumulation was decreased in I/R-injured venules of GF mice, NETosis following I/R injury was significantly enhanced compared with conventionally raised mice or mice colonized with the minimal microbial consortium altered Schaedler flora. Also ex vivo, neutrophils from GF and antibiotic-treated mice showed increased LPS (lipopolysaccharide)-induced NETosis. Enhanced TLR4 signaling in GF neutrophils was due to elevated TLR4 expression and augmented IRF3 (interferon regulatory factor-3) phosphorylation. Likewise, neutrophils from antibiotic-treated conventionally raised mice had increased NET formation before and after ischemia. Increased NETosis in I/R injury was abolished in conventionally raised mice deficient in the TLR adaptor TRIF. In support of the desensitizing influence of enteric LPS, treatment of GF mice with LPS via drinking water diminished LPS-induced NETosis in vitro and in the mesenteric I/R injury model.
Conclusions: Collectively, our results identified that the gut microbiota suppresses NETing neutrophil hyperreactivity in mesenteric I/R injury, while ensuring immunovigilance by enhancing neutrophil recruitment.
Keywords: extracellular traps; interferons; lipopolysaccharide; neutrophils; venules.
Conflict of interest statement
Disclosures
The authors declare no conflicts of interest.
Figures




Comment in
-
Gut Feeling: The Role of Gut Microbiota in Immunomodulation of Ischemia-Reperfusion Injury.Arterioscler Thromb Vasc Biol. 2020 Sep;40(9):1967-1969. doi: 10.1161/ATVBAHA.120.314941. Epub 2020 Aug 26. Arterioscler Thromb Vasc Biol. 2020. PMID: 32845773 No abstract available.
-
Letter by Xiao et al Regarding Article, "Gut Microbiota Restricts NETosis in Acute Mesenteric Ischemia-Reperfusion Injury".Arterioscler Thromb Vasc Biol. 2021 Jan;41(1):e72-e73. doi: 10.1161/ATVBAHA.120.315532. Epub 2020 Dec 23. Arterioscler Thromb Vasc Biol. 2021. PMID: 33356368 No abstract available.
-
Response by Ascher et al to Letter Regarding Article, "Gut Microbiota Restricts NETosis in Acute Mesenteric Ischemia-Reperfusion Injury".Arterioscler Thromb Vasc Biol. 2021 Jan;41(1):e74-e75. doi: 10.1161/ATVBAHA.120.315541. Epub 2020 Dec 23. Arterioscler Thromb Vasc Biol. 2021. PMID: 33356371 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

