Phyllanthus amarus prevents LPS-mediated BV2 microglial activation via MyD88 and NF-κB signaling pathways
- PMID: 32611404
- PMCID: PMC7330992
- DOI: 10.1186/s12906-020-02961-0
Phyllanthus amarus prevents LPS-mediated BV2 microglial activation via MyD88 and NF-κB signaling pathways
Abstract
Background: Phyllanthus amarus has been shown to attenuate lipopolysaccharide (LPS)-induced peripheral inflammation but similar studies in the central nervous system are scarce. The aim of the present study was to investigate the neuroprotective effects of 80% ethanol extract of P. amarus (EPA) in LPS-activated BV2 microglial cells.
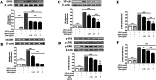
Methods: BV2 microglial cells c for 24 h, pre-treated with EPA for 24 h prior to LPS induction for another 24 h. Surface expression of CD11b and CD40 on BV2 cells was analyzed by flow cytometry. ELISA was employed to measure the production of pro-inflammatory mediators i.e. nitric oxide (NO) and tumor necrosis factor (TNF)-α. Western blotting technique was used to determine the expression of inducible nitric oxide synthase (iNOS), myeloid differentiation protein 88 (MYD88), nuclear factor kappa B (NF-κB), caspase-1, and mitogen activated protein kinase (MAPK).
Results: Qualitative and quantitative analyses of the EPA using a validated ultra-high pressure liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) method indicated the presence of phyllanthin, hypophyllanthin, niranthin, ellagic acid, corilagin, gallic acid, phyltetralin, isolintetralin and geraniin. EPA suppressed the production of NO and TNFα in LPS-activated BV2 microglial cells. Moreover, EPA attenuated the expression of MyD88, NF-κB and MAPK (p-P38, p-JNK and p-ERK1/2). It also inhibited the expression of CD11b and CD40. EPA protected against LPS-induced microglial activation via MyD88 and NF-κB signaling in BV2 microglial cells.
Conclusions: EPA demonstrated neuroprotective effects against LPS-induced microglial cells activation through the inhibition of TNFα secretion, iNOS protein expression and subsequent NO production, inhibition of NF-κB and MAPKs mediated by adapter protein MyD88 and inhibition of microglial activation markers CD11b and CD40.
Keywords: BV2 microglial cells; Microglial activation; Neuroinflammation; Neuroprotection; Phyllanthus amarus.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



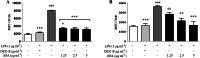
References
-
- Wang X, Hu D, Zhang L, Lian G, Zhao S, Wang C, et al. Gomisin A inhibits lipopolysaccharide-induced inflammatory responses in N9 microglia via blocking the NF-κB/MAPKs pathway. Food Chem Toxicol. 2014;63:119–127. - PubMed
-
- Kofler J, Wiley CA. Microglia. Toxicol Pathol. 2011;39(1):103–114. - PubMed
-
- Labzin LI, Heneka MT, Latz E. Innate Immunity and Neurodegeneration. Annu Rev Med. 2018;69(1):437–449. - PubMed
-
- Kettenmann H, Hanisch U, Noda M, Verkhratsky A. Physiology of Microglia. Physiol Rev. 2011;91:461–553. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

