The radiobiology of HPV-positive and HPV-negative head and neck squamous cell carcinoma
- PMID: 32611474
- PMCID: PMC7754878
- DOI: 10.1017/erm.2020.4
The radiobiology of HPV-positive and HPV-negative head and neck squamous cell carcinoma
Abstract
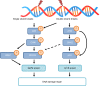
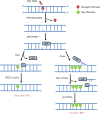
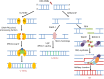
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide, with reported incidences of ~800 000 cases each year. One of the critical determinants in patient response to radiotherapy, particularly for oropharyngeal cancers, is human papillomavirus (HPV) status where HPV-positive patients display improved survival rates and outcomes particularly because of increased responsiveness to radiotherapy. The increased radiosensitivity of HPV-positive HNSCC has been largely linked with defects in the signalling and repair of DNA double-strand breaks. Therefore, strategies to further radiosensitise HPV-positive HNSCC, but also radioresistant HPV-negative HNSCC, have focussed on targeting key DNA repair proteins including PARP, DNA-Pk, ATM and ATR. However, inhibitors against CHK1 and WEE1 involved in cell-cycle checkpoint activation have also been investigated as targets for radiosensitisation in HNSCC. These studies, largely conducted using established HNSCC cell lines in vitro, have demonstrated variability in the response dependent on the specific inhibitors and cell models utilised. However, promising results are evident targeting specifically PARP, DNA-Pk, ATR and CHK1 in synergising with radiation in HNSCC cell killing. Nevertheless, these preclinical studies require further expansion and investigation for translational opportunities for the effective treatment of HNSCC in combination with radiotherapy.
Keywords: DNA damage; DNA repair; head and neck cancer; human papillomavirus; ionising radiation; radiation biology.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
References
-
- Bray F, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal 68, 394–424. - PubMed
-
- Forastiere A et al. (2001) Head and neck cancer. New England Journal of Medicine 345, 1890–1900. - PubMed
-
- Lajer CB and von Buchwald C (2010) The role of human papillomavirus in head and neck cancer. APMIS 118, 510–519. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

