Non-adherence in non-inferiority trials: pitfalls and recommendations
- PMID: 32611541
- PMCID: PMC7327542
- DOI: 10.1136/bmj.m2215
Non-adherence in non-inferiority trials: pitfalls and recommendations
Erratum in
-
Non-adherence in non-inferiority trials: pitfalls and recommendations.BMJ. 2020 Jul 10;370:m2692. doi: 10.1136/bmj.m2692. BMJ. 2020. PMID: 32651165 Free PMC article. No abstract available.
Abstract
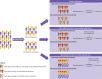
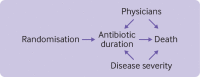
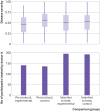
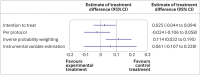
Non-adherence in non-inferiority trials can affect treatment effect estimates and often increases the chance of claiming non-inferiority under the standard intention-to-treat analysis. This article discusses the implications of different patterns of non-adherence in non-inferiority trials and offers practical recommendations for trial design, alternative analysis strategies, and outcome reporting to reduce bias in treatment estimates and improve transparency in reporting.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the Singapore National Medical Research Council and the UK Medical Research Council/Department for International Development for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. Provenance and peer review: Not commissioned; externally peer reviewed.
Figures

Similar articles
-
Non-inferiority trials in cardiology: what clinicians need to know.Heart. 2020 Jan;106(2):99-104. doi: 10.1136/heartjnl-2019-315772. Epub 2019 Oct 31. Heart. 2020. PMID: 31672779 Free PMC article. Review.
-
Reporting non-adherence in cluster randomised trials: A systematic review.Clin Trials. 2018 Jun;15(3):294-304. doi: 10.1177/1740774518761666. Epub 2018 Apr 2. Clin Trials. 2018. PMID: 29608096 Free PMC article.
-
Applying the Estimands Framework to Non-Inferiority Trials: Guidance on Choice of Hypothetical Estimands for Non-Adherence and Comparison of Estimation Methods.Stat Med. 2025 Feb 28;44(5):e10348. doi: 10.1002/sim.10348. Stat Med. 2025. PMID: 39921280 Free PMC article.
-
Superiority and non-inferiority: two sides of the same coin?Trials. 2018 Sep 17;19(1):499. doi: 10.1186/s13063-018-2885-z. Trials. 2018. PMID: 30223881 Free PMC article.
-
Exploring how non-inferiority and equivalence are assessed in paediatrics: a systematic review.Arch Dis Child. 2018 Nov;103(11):1067-1075. doi: 10.1136/archdischild-2018-314874. Epub 2018 May 24. Arch Dis Child. 2018. PMID: 29794107
Cited by
-
Statistical methods for non-adherence in non-inferiority trials: useful and used? A systematic review.BMJ Open. 2022 Jan 12;12(1):e052656. doi: 10.1136/bmjopen-2021-052656. BMJ Open. 2022. PMID: 35022173 Free PMC article.
-
Key insights and challeneges in noninferiority trials.Korean J Anesthesiol. 2024 Aug;77(4):423-431. doi: 10.4097/kja.23534. Epub 2024 Jul 30. Korean J Anesthesiol. 2024. PMID: 39081188 Free PMC article. Review.
-
Methodological Challenges in Randomized Controlled Trials of mHealth Interventions: Cross-Sectional Survey Study and Consensus-Based Recommendations.J Med Internet Res. 2024 Dec 19;26:e53187. doi: 10.2196/53187. J Med Internet Res. 2024. PMID: 39700488 Free PMC article.
-
Weaning of maintenance immunosuppressive therapy in lupus nephritis (WIN-Lupus): results of a multicentre randomised controlled trial.Ann Rheum Dis. 2022 Oct;81(10):1420-1427. doi: 10.1136/annrheumdis-2022-222435. Epub 2022 Jun 20. Ann Rheum Dis. 2022. PMID: 35725295 Free PMC article. Clinical Trial.
-
Non-efficacy benefits and non-inferiority margins: a scoping review of contemporary high-impact non-inferiority trials in clinical cardiology.Eur J Epidemiol. 2021 Nov;36(11):1103-1109. doi: 10.1007/s10654-021-00820-x. Epub 2021 Nov 18. Eur J Epidemiol. 2021. PMID: 34792692 Free PMC article. No abstract available.
References
-
- US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER). Non-inferiority clinical trials to establish effectiveness-guidance for industry. 2016. https://www.fda.gov/downloads/Drugs/Guidances/UCM202140.pdf.
-
- Standards of care - Nuffield Bioethics. Nuffield Bioethics. https://nuffieldbioethics.org/report/research-developing-countries-2/sta....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
