Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis
- PMID: 32611558
- PMCID: PMC7327913
- DOI: 10.1136/bmj.m2516
Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis
Abstract
Objective: To determine the diagnostic accuracy of serological tests for coronavirus disease-2019 (covid-19).
Design: Systematic review and meta-analysis.
Data sources: Medline, bioRxiv, and medRxiv from 1 January to 30 April 2020, using subject headings or subheadings combined with text words for the concepts of covid-19 and serological tests for covid-19.
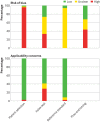
Eligibility criteria and data analysis: Eligible studies measured sensitivity or specificity, or both of a covid-19 serological test compared with a reference standard of viral culture or reverse transcriptase polymerase chain reaction. Studies were excluded with fewer than five participants or samples. Risk of bias was assessed using quality assessment of diagnostic accuracy studies 2 (QUADAS-2). Pooled sensitivity and specificity were estimated using random effects bivariate meta-analyses.
Main outcome measures: The primary outcome was overall sensitivity and specificity, stratified by method of serological testing (enzyme linked immunosorbent assays (ELISAs), lateral flow immunoassays (LFIAs), or chemiluminescent immunoassays (CLIAs)) and immunoglobulin class (IgG, IgM, or both). Secondary outcomes were stratum specific sensitivity and specificity within subgroups defined by study or participant characteristics, including time since symptom onset.
Results: 5016 references were identified and 40 studies included. 49 risk of bias assessments were carried out (one for each population and method evaluated). High risk of patient selection bias was found in 98% (48/49) of assessments and high or unclear risk of bias from performance or interpretation of the serological test in 73% (36/49). Only 10% (4/40) of studies included outpatients. Only two studies evaluated tests at the point of care. For each method of testing, pooled sensitivity and specificity were not associated with the immunoglobulin class measured. The pooled sensitivity of ELISAs measuring IgG or IgM was 84.3% (95% confidence interval 75.6% to 90.9%), of LFIAs was 66.0% (49.3% to 79.3%), and of CLIAs was 97.8% (46.2% to 100%). In all analyses, pooled sensitivity was lower for LFIAs, the potential point-of-care method. Pooled specificities ranged from 96.6% to 99.7%. Of the samples used for estimating specificity, 83% (10 465/12 547) were from populations tested before the epidemic or not suspected of having covid-19. Among LFIAs, pooled sensitivity of commercial kits (65.0%, 49.0% to 78.2%) was lower than that of non-commercial tests (88.2%, 83.6% to 91.3%). Heterogeneity was seen in all analyses. Sensitivity was higher at least three weeks after symptom onset (ranging from 69.9% to 98.9%) compared with within the first week (from 13.4% to 50.3%).
Conclusion: Higher quality clinical studies assessing the diagnostic accuracy of serological tests for covid-19 are urgently needed. Currently, available evidence does not support the continued use of existing point-of-care serological tests.
Study registration: PROSPERO CRD42020179452.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: SL reports personal fees from Carebook Technologies, outside the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
Comment in
-
Antibody testing for coronavirus disease 2019: not ready for prime time.BMJ. 2020 Jul 3;370:m2655. doi: 10.1136/bmj.m2655. BMJ. 2020. PMID: 32620675 Free PMC article.
References
-
- American Society for Microbiology. ASM expresses concern about coronavirus test reagent shortages. https://asm.org/Articles/Policy/2020/March/ASM-Expresses-Concern-about-T.... 2020
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous