Adverse Drug Reactions in Patients with CKD
- PMID: 32611662
- PMCID: PMC7409761
- DOI: 10.2215/CJN.01030120
Adverse Drug Reactions in Patients with CKD
Abstract
Background and objectives: Little is known about the burden of adverse drug reactions in CKD. We estimated the incidence of overall and serious adverse drug reactions and assessed the probability of causation, preventability, and factors associated with adverse drug reactions in patients seen by nephrologists.
Design, setting, participants, & measurements: The Chronic Kidney Disease-Renal Epidemiology and Information Network cohort included 3033 outpatients (65% men) with CKD and eGFR<60 ml/min per 1.73 m2, with follow-up for 2 years. Adverse drug reactions were identified from hospitalization reports, medical records, and participant interviews and finally assessed for causality, preventability, and immediate therapeutic management by experts in pharmacology.
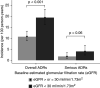
Results: Median (interquartile range) age was 69 (60-76) years old; 55% had eGFR≥30 ml/min per 1.73 m2, and 45% had eGFR<30 ml/min per 1.73 m2. Participants were prescribed a median (range) of eight (five to ten) drugs. Over 2 years, 536 patients had 751 adverse drug reactions, 150 (in 125 participants) classified as serious, for rates of 14.4 (95% confidence interval, 12.6 to 16.5) and 2.7 (95% confidence interval, 1.7 to 4.3) per 100 person-years, respectively. Among the serious adverse drug reactions, 32% were considered preventable or potentially preventable; 16 caused death, directly or indirectly. Renin-angiotensin system inhibitors (15%), antithrombotic agents (14%), and diuretics (10%) were the drugs to which the most adverse drug reactions were imputed, but antithrombotic agents caused 34% of serious adverse drug reactions. The drug was discontinued in 71% of cases, at least temporarily. Adjusted hazard ratios for serious adverse drug reaction were significantly higher in patients with eGFR<30 versus ≥30 ml/min per 1.73 m2 (1.8; 95% confidence interval, 1.3 to 2.6), in those prescribed more than ten versus less than five medications (2.4; 95% confidence interval, 1.1 to 5.2), or in those with poor versus good adherence (1.6; 95% confidence interval, 1.4 to 2.4).
Conclusions: Adverse drug reactions are common and sometimes serious in patients with CKD. Many serious adverse drug reactions may be preventable. Some specific pharmacologic classes, particularly antithrombotic agents, are at risk of serious adverse drug reactions.
Clinical trial registry name and registration number: Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN), NCT03381950.
Keywords: Chronic; Cohort Studies; Drug-Related Side Effects and Adverse Reactions; Medical Records; Renal Insufficiency; Renin-Angiotensin System; adverse drug reactions; antithrombotic agents; chronic kidney disease; diuretics; glomerular filtration rate; hospitalization; pharmacoepidemiology; risk factors.
Copyright © 2020 by the American Society of Nephrology.
Figures



Comment in
-
Adverse Drug Effects in Patients with CKD: Primum Non Nocere.Clin J Am Soc Nephrol. 2020 Aug 7;15(8):1075-1077. doi: 10.2215/CJN.08890620. Epub 2020 Jul 1. Clin J Am Soc Nephrol. 2020. PMID: 32611663 Free PMC article. No abstract available.
References
-
- Lazarou J, Pomeranz BH, Corey PN: Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA 279: 1200–1205, 1998. - PubMed
-
- Kongkaew C, Noyce PR, Ashcroft DM: Hospital admissions associated with adverse drug reactions: A systematic review of prospective observational studies. Ann Pharmacother 42: 1017–1025, 2008. - PubMed
-
- Taché SV, Sönnichsen A, Ashcroft DM: Prevalence of adverse drug events in ambulatory care: A systematic review. Ann Pharmacother 45: 977–989, 2011. - PubMed
-
- Bénard-Laribière A, Miremont-Salamé G, Pérault-Pochat M-C, Noize P, Haramburu F; EMIR Study Group on behalf of the French network of pharmacovigilance centres : Incidence of hospital admissions due to adverse drug reactions in France: The EMIR study. Fundam Clin Pharmacol 29: 106–111, 2015. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

