Utility of Stool PCR for the Diagnosis of COVID-19: Comparison of Two Commercial Platforms
- PMID: 32611796
- PMCID: PMC7448643
- DOI: 10.1128/JCM.01369-20
Utility of Stool PCR for the Diagnosis of COVID-19: Comparison of Two Commercial Platforms
Abstract
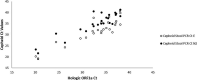
The ability to detect SARS-CoV-2 in the upper respiratory tract ceases after 2 to 3 weeks post-symptom-onset in most patients. In contrast, SARS-CoV-2 can be detected in the stool of some patients for greater than 4 weeks, suggesting that stool may hold utility as an additional source for diagnosis. We validated the Cepheid Xpert Xpress SARS-CoV-2 and Hologic Panther Fusion real-time RT-PCR assays for detection of viral RNA in stool specimens and compared performance. We utilized remnant stool specimens (n = 79) from 77 patients with gastrointestinal symptoms. Forty-eight patients had PCR-confirmed COVID-19, and 29 either were nasopharyngeal/oropharyngeal PCR negative or presented for reasons unrelated to COVID-19 and were not tested. Positive percent agreement between the Cepheid and Hologic assays was 93% (95% confidence interval [CI]: 81.1% to 98.2%), and negative percent agreement was 96% (95% CI: 89% to 0.99%). Four discrepant specimens (Cepheid positive only, n = 2; Hologic positive only, n = 2) exhibited average cycle threshold (CT ) values of >37 for the targets detected. Of the 48 patients with PCR-confirmed COVID-19, 23 were positive by both assays (47.9%). For the negative patient group, 2/29 were positive by both assays (6.9%). The two stool PCR-positive, nasopharyngeal/oropharyngeal PCR-negative patients were SARS-CoV-2 IgG positive. Our results demonstrate acceptable agreement between two commercially available molecular assays and support the use of stool PCR to confirm diagnosis when SARS-CoV-2 is undetectable in the upper respiratory tract.
Keywords: COVID-19; SARS-CoV-2; diagnostics; stool PCR.
Copyright © 2020 American Society for Microbiology.
Figures

References
-
- Wolfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Muller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brunink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. 2020. Virological assessment of hospitalized patients with COVID-2019. Nature 581:465–469. doi:10.1038/s41586-020-2196-x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

