Development of indigenous IgG ELISA for the detection of anti-SARS-CoV-2 IgG
- PMID: 32611915
- PMCID: PMC7530443
- DOI: 10.4103/ijmr.IJMR_2232_20
Development of indigenous IgG ELISA for the detection of anti-SARS-CoV-2 IgG
Abstract
Background & objectives: Since the beginning of the year 2020, the pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) impacted humankind adversely in almost all spheres of life. The virus belongs to the genus Betacoronavirus of the family Coronaviridae. SARS-CoV-2 causes the disease known as coronavirus disease 2019 (COVID-19) with mild-to-severe respiratory illness. The currently available diagnostic tools for the diagnosis of COVID-19 are mainly based on molecular assays. Real-time reverse transcription-polymerase chain reaction is the only diagnostic method currently recommended by the World Health Organization for COVID-19. With the rapid spread of SARS-CoV-2, it is necessary to utilize other tests, which would determine the burden of the disease as well as the spread of the outbreak. Considering the need for the development of such a screening test, an attempt was made to develop and evaluate an IgG-based ELISA for COVID-19.
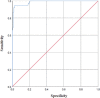
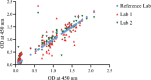
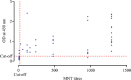
Methods: A total of 513 blood samples (131 positive, 382 negative for SARS-CoV-2) were collected and tested by microneutralization test (MNT). Antigen stock of SARS-CoV-2 was prepared by propagating the virus in Vero CCL-81 cells. An IgG capture ELISA was developed for serological detection of anti-SARS-CoV-2 IgG in serum samples. The end point cut-off values were determined by using receiver operating characteristic (ROC) curve. Inter-assay variability was determined.
Results: The developed ELISA was found to be 92.37 per cent sensitive, 97.9 per cent specific, robust and reproducible. The positive and negative predictive values were 94.44 and 98.14 per cent, respectively.
Interpretation & conclusions: This indigenously developed IgG ELISA was found to be sensitive and specific for the detection of anti-SARS-CoV-2 IgG in human serum samples. This assay may be used for determining seroprevalence of SARS-CoV-2 in a population exposed to the virus.
Keywords: COVID-19 - diagnosis - ELISA - human - IgG antibodies - microneutralization test - SARS-CoV-2 - standardization.
Conflict of interest statement
None
Figures
References
-
- World Health Organization. WHO coronavirus disease (COVID-19) Dashboard. [accessed on May 27, 2020]. Available from: https://covid19whoint/">https://covid19whoint/
-
- Holmes KV. Coronaviruses (Coronaviridae) Encyclopedia Virol. 1999:291–8.
-
- Hamre D, Procknow JJ. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 1966;121:190–3. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
