Injury intensifies T cell mediated graft-versus-host disease in a humanized model of traumatic brain injury
- PMID: 32612177
- PMCID: PMC7330041
- DOI: 10.1038/s41598-020-67723-x
Injury intensifies T cell mediated graft-versus-host disease in a humanized model of traumatic brain injury
Abstract
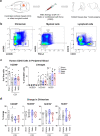
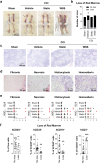
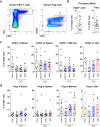
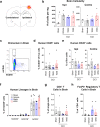
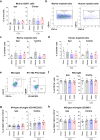
The immune system plays critical roles in promoting tissue repair during recovery from neurotrauma but is also responsible for unchecked inflammation that causes neuronal cell death, systemic stress, and lethal immunodepression. Understanding the immune response to neurotrauma is an urgent priority, yet current models of traumatic brain injury (TBI) inadequately recapitulate the human immune response. Here, we report the first description of a humanized model of TBI and show that TBI places significant stress on the bone marrow. Hematopoietic cells of the marrow are regionally decimated, with evidence pointing to exacerbation of underlying graft-versus-host disease (GVHD) linked to presence of human T cells in the marrow. Despite complexities of the humanized mouse, marrow aplasia caused by TBI could be alleviated by cell therapy with human bone marrow mesenchymal stromal cells (MSCs). We conclude that MSCs could be used to ameliorate syndromes triggered by hypercytokinemia in settings of secondary inflammatory stimulus that upset marrow homeostasis such as TBI. More broadly, this study highlights the importance of understanding how underlying immune disorders including immunodepression, autoimmunity, and GVHD might be intensified by injury.
Conflict of interest statement
C.S.C. serves on the Scientific Advisory Board for CBR, Inc. and conducts sponsored research with Athersys, Inc., Celgene Therapeutics, Inc., and CBR. K.R.A, B.S.G., C.S.C., and P.L.W. are inventors on a patent for conditioning of stem and progenitor cells for cellular therapy (U.S. Patent US20180187141A1). All other authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

