Structure and flexibility in cortical representations of odour space
- PMID: 32612230
- PMCID: PMC7450987
- DOI: 10.1038/s41586-020-2451-1
Structure and flexibility in cortical representations of odour space
Erratum in
-
Author Correction: Structure and flexibility in cortical representations of odour space.Nature. 2020 Aug;584(7822):E38. doi: 10.1038/s41586-020-2615-z. Nature. 2020. PMID: 32782391
Abstract
The cortex organizes sensory information to enable discrimination and generalization1-4. As systematic representations of chemical odour space have not yet been described in the olfactory cortex, it remains unclear how odour relationships are encoded to place chemically distinct but similar odours, such as lemon and orange, into perceptual categories, such as citrus5-7. Here, by combining chemoinformatics and multiphoton imaging in the mouse, we show that both the piriform cortex and its sensory inputs from the olfactory bulb represent chemical odour relationships through correlated patterns of activity. However, cortical odour codes differ from those in the bulb: cortex more strongly clusters together representations for related odours, selectively rewrites pairwise odour relationships, and better matches odour perception. The bulb-to-cortex transformation depends on the associative network originating within the piriform cortex, and can be reshaped by passive odour experience. Thus, cortex actively builds a structured representation of chemical odour space that highlights odour relationships; this representation is similar across individuals but remains plastic, suggesting a means through which the olfactory system can assign related odour cues to common and yet personalized percepts.
Conflict of interest statement
Authors declare no competing interests.
Figures

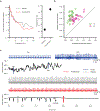



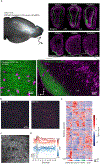
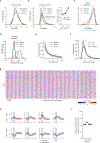
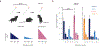





Similar articles
-
Spatial maps in piriform cortex during olfactory navigation.Nature. 2022 Jan;601(7894):595-599. doi: 10.1038/s41586-021-04242-3. Epub 2021 Dec 22. Nature. 2022. PMID: 34937941
-
Odour recognition and segmentation by a model olfactory bulb and cortex.Network. 2000 Feb;11(1):83-102. Network. 2000. PMID: 10735530
-
Acquisition of non-olfactory encoding improves odour discrimination in olfactory cortex.Nat Commun. 2024 Jul 2;15(1):5572. doi: 10.1038/s41467-024-49897-4. Nat Commun. 2024. PMID: 38956072 Free PMC article.
-
Bilateral and unilateral odor processing and odor perception.Commun Biol. 2020 Apr 1;3(1):150. doi: 10.1038/s42003-020-0876-6. Commun Biol. 2020. PMID: 32238904 Free PMC article. Review.
-
Complex neural representation of odour information in the olfactory bulb.Acta Physiol (Oxf). 2020 Jan;228(1):e13333. doi: 10.1111/apha.13333. Epub 2019 Jul 2. Acta Physiol (Oxf). 2020. PMID: 31188539 Free PMC article. Review.
Cited by
-
Smell-induced gamma oscillations in human olfactory cortex are required for accurate perception of odor identity.PLoS Biol. 2022 Jan 5;20(1):e3001509. doi: 10.1371/journal.pbio.3001509. eCollection 2022 Jan. PLoS Biol. 2022. PMID: 34986157 Free PMC article.
-
Distinct information conveyed to the olfactory bulb by feedforward input from the nose and feedback from the cortex.Nat Commun. 2024 Apr 16;15(1):3268. doi: 10.1038/s41467-024-47366-6. Nat Commun. 2024. PMID: 38627390 Free PMC article.
-
Representational learning by optimization of neural manifolds in an olfactory memory network.Res Sq [Preprint]. 2025 Mar 26:rs.3.rs-6155477. doi: 10.21203/rs.3.rs-6155477/v1. Res Sq. 2025. PMID: 40195987 Free PMC article. Preprint.
-
A biological model of nonlinear dimensionality reduction.Sci Adv. 2025 Feb 7;11(6):eadp9048. doi: 10.1126/sciadv.adp9048. Epub 2025 Feb 5. Sci Adv. 2025. PMID: 39908371 Free PMC article.
-
High-precision mapping reveals the structure of odor coding in the human brain.Nat Neurosci. 2023 Sep;26(9):1595-1602. doi: 10.1038/s41593-023-01414-4. Epub 2023 Aug 24. Nat Neurosci. 2023. PMID: 37620443 Free PMC article.
References
-
- Haberly LB Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chemical Senses 26, 551–576 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

