Clinical targeting of HIV capsid protein with a long-acting small molecule
- PMID: 32612233
- PMCID: PMC8188729
- DOI: 10.1038/s41586-020-2443-1
Clinical targeting of HIV capsid protein with a long-acting small molecule
Abstract
Oral antiretroviral agents provide life-saving treatments for millions of people living with HIV, and can prevent new infections via pre-exposure prophylaxis1-5. However, some people living with HIV who are heavily treatment-experienced have limited or no treatment options, owing to multidrug resistance6. In addition, suboptimal adherence to oral daily regimens can negatively affect the outcome of treatment-which contributes to virologic failure, resistance generation and viral transmission-as well as of pre-exposure prophylaxis, leading to new infections1,2,4,7-9. Long-acting agents from new antiretroviral classes can provide much-needed treatment options for people living with HIV who are heavily treatment-experienced, and additionally can improve adherence10. Here we describe GS-6207, a small molecule that disrupts the functions of HIV capsid protein and is amenable to long-acting therapy owing to its high potency, low in vivo systemic clearance and slow release kinetics from the subcutaneous injection site. Drawing on X-ray crystallographic information, we designed GS-6207 to bind tightly at a conserved interface between capsid protein monomers, where it interferes with capsid-protein-mediated interactions between proteins that are essential for multiple phases of the viral replication cycle. GS-6207 exhibits antiviral activity at picomolar concentrations against all subtypes of HIV-1 that we tested, and shows high synergy and no cross-resistance with approved antiretroviral drugs. In phase-1 clinical studies, monotherapy with a single subcutaneous dose of GS-6207 (450 mg) resulted in a mean log10-transformed reduction of plasma viral load of 2.2 after 9 days, and showed sustained plasma exposure at antivirally active concentrations for more than 6 months. These results provide clinical validation for therapies that target the functions of HIV capsid protein, and demonstrate the potential of GS-6207 as a long-acting agent to treat or prevent infection with HIV.
Figures

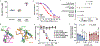
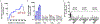

References
Main References
-
- Thigpen MC et al. Antiretroviral Preexposure Prophylaxis for Heterosexual HIV Transmission in Botswana [Supplementary Appendix]. N Eng J Med 367, 423–434 (2012). - PubMed
-
- World Health Organization (WHO). HIV/AIDS fact sheets. https://www.who.int/news-room/fact-sheets/detail/hiv-aids. November 15 (2019).
Methods Only References
-
- Nakabayashi H, Taketa K, Miyano K, Yamane T & Sato J Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res 42, 3858–3863 (1982). - PubMed
-
- Prichard MN & Shipman C Jr. Analysis of combinations of antiviral drugs and design of effective multidrug therapies. Antivir Ther 1, 9–20 (1996). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

