One Is Not Enough: Understanding and Modeling Polysubstance Use
- PMID: 32612502
- PMCID: PMC7309369
- DOI: 10.3389/fnins.2020.00569
One Is Not Enough: Understanding and Modeling Polysubstance Use
Abstract
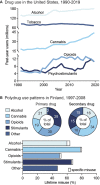
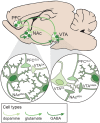
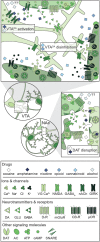
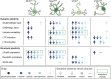
Substance use disorder (SUD) is a chronic, relapsing disease with a highly multifaceted pathology that includes (but is not limited to) sensitivity to drug-associated cues, negative affect, and motivation to maintain drug consumption. SUDs are highly prevalent, with 35 million people meeting criteria for SUD. While drug use and addiction are highly studied, most investigations of SUDs examine drug use in isolation, rather than in the more prevalent context of comorbid substance histories. Indeed, 11.3% of individuals diagnosed with a SUD have concurrent alcohol and illicit drug use disorders. Furthermore, having a SUD with one substance increases susceptibility to developing dependence on additional substances. For example, the increased risk of developing heroin dependence is twofold for alcohol misusers, threefold for cannabis users, 15-fold for cocaine users, and 40-fold for prescription misusers. Given the prevalence and risk associated with polysubstance use and current public health crises, examining these disorders through the lens of co-use is essential for translatability and improved treatment efficacy. The escalating economic and social costs and continued rise in drug use has spurred interest in developing preclinical models that effectively model this phenomenon. Here, we review the current state of the field in understanding the behavioral and neural circuitry in the context of co-use with common pairings of alcohol, nicotine, cannabis, and other addictive substances. Moreover, we outline key considerations when developing polysubstance models, including challenges to developing preclinical models to provide insights and improve treatment outcomes.
Keywords: addiction; neurobiology of addiction; neuronal signaling and behavior; polydrug; preclinical models; review; reward circuitry; substance use.
Copyright © 2020 Crummy, O’Neal, Baskin and Ferguson.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

