Sex-Specific Response to Caloric Restriction After Reproductive Investment in Microcebus murinus: An Integrative Approach
- PMID: 32612534
- PMCID: PMC7308708
- DOI: 10.3389/fphys.2020.00506
Sex-Specific Response to Caloric Restriction After Reproductive Investment in Microcebus murinus: An Integrative Approach
Abstract
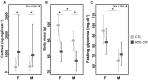
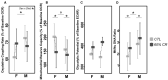
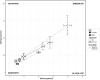
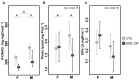
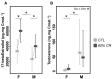
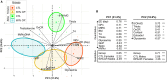
In seasonal environments, males and females usually maintain high metabolic activity during the whole summer season, exhausting their energy reserves. In the global warming context, unpredictability of food availability during summer could dramatically challenge the energy budget of individuals. Therefore, one can predict that resilience to environmental stress would be dramatically endangered during summer. Here, we hypothesized that females could have greater capacity to survive harsh conditions than males, considering the temporal shift in their respective reproductive energy investment, which can challenge them differently, as well as enhanced flexibility in females' physiological regulation. We tackled this question on the gray mouse lemur (Microcebus murinus), focusing on the late summer period, after the reproductive effort. We monitored six males and six females before and after a 2-weeks 60% caloric restriction (CR), measuring different physiological and cellular parameters in an integrative and comparative multiscale approach. Before CR, females were heavier than males and mostly characterized by high levels of energy expenditure, a more energetic mitochondrial profile and a downregulation of blood antioxidants. We observed a similar energy balance between sexes due to CR, with a decrease in metabolic activity over time only in males. Oxidative damage to DNA was also reduced by different pathways between sexes, which may reflect variability in their physiological status and life-history traits at the end of summer. Finally, females' mitochondria seemed to exhibit greater flexibility and greater metabolic potential than males in response to CR. Our results showed strong differences between males and females in response to food shortage during late summer, underlining the necessity to consider sex as a factor for population dynamics in climate change models.
Keywords: caloric restriction; non-human primate; oxidative stress; reproductive investment; season; sex.
Copyright © 2020 Noiret, Puch, Riffaud, Costantini, Riou, Aujard and Terrien.
Figures


References
-
- Aslam H., Schneiders A., Perret M., Weinbauer G. F., Hodges J. K. (2002). Quantitative assessment of testicular germ cell production and kinematic and morphometric parameters of ejaculated spermatozoa in the grey mouse lemur, Microcebus murinus. Reproduction 123, 323–332. 10.1530/rep.0.1230323 - DOI - PubMed
-
- Bieber C., Lebl K., Stalder G., Geiser F., Ruf T. (2014). Body mass dependent use of hibernation: why not prolong the active season, if they can? Funct. Ecol. 28, 167–177. 10.1111/1365-2435.12173 - DOI
-
- Breuner C. W., Delehanty B., Boonstra R. (2013). Evaluating stress in natural populations of vertebrates: total CORT is not good enough. Funct. Ecol. 27, 24–36. 10.1111/1365-2435.12016 - DOI
LinkOut - more resources
Full Text Sources

