Are the Effects of Oral and Vaginal Contraceptives on Bone Formation in Young Women Mediated via the Growth Hormone-IGF-I Axis?
- PMID: 32612574
- PMCID: PMC7309348
- DOI: 10.3389/fendo.2020.00334
Are the Effects of Oral and Vaginal Contraceptives on Bone Formation in Young Women Mediated via the Growth Hormone-IGF-I Axis?
Abstract
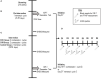
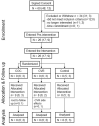
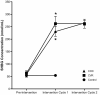
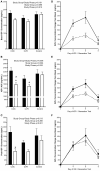
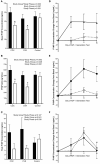
Purpose: Combined hormonal contraceptive therapy has been associated with negative bone mineral density outcomes that may be route-dependent [i.e., combined oral contraception (COC) vs. contraceptive vaginal ring (CVR)] and involve the hepatic growth hormone (GH)/insulin-like growth factor-I (IGF-I) axis. The objective of the pilot study was to assess the impact of route of contraceptive administration on IGF-I and procollagen type I N-terminal propeptide (PINP) responses to an IGF-I Generation Test. We hypothesized that the peak rise in IGF-I and PINP concentration and area under the curve (AUC) would be attenuated following COC, but not CVR, use. Methods: Healthy, premenopausal women not taking hormonal contraception were recruited. Women were enrolled in the control group (n = 8) or randomly assigned to COC (n = 8) or CVR (n = 8) for two contraceptive cycles. IGF-I Generation Tests were used as a probe to stimulate IGF-I release and were completed during the pre-intervention and intervention phases. Serum IGF-I and PINP were measured during both IGF-I Generation Tests. The study was registered at ClinicalTrials.gov (NCT02367833). Results: Compared to the pre-intervention phase, peak IGF-I concentration in response to the IGF-I Generation Test in the intervention phase was suppressed in the COC group (p < 0.001), but not the CVR or Control groups (p > 0.090). Additionally, compared to the pre-intervention phase, PINP AUC during the intervention phase was suppressed in both COC and CVR groups (p < 0.001), while no difference was observed in the control group (p = 0.980). Conclusion: These data suggest that changes in recombinant human GH-stimulated hepatic IGF-I synthesis in response to combined hormonal contraception (CHC) use are dependent on route of CHC administration, while the influence on PINP is route-independent. Future research is needed to expand these results with larger randomized control trials in all age ranges of women who utilize hormonal contraception. Clinical Trial Registration: www.ClinicalTrials.gov registration NCT02367833.
Keywords: IGF-I generation test; contraceptive vaginal ring; insulin-like growth factor-I; oral contraception; procollagen type I N-terminal propeptide.
Copyright © 2020 Allaway, Misra, Southmayd, Stone, Weaver, Petkus and De Souza.
Figures
References
-
- Daniels K, Abma JC. Current Contraceptive Status Among Women Aged 15–49: United States, 2015–2017. NCHS Data Brief, no 327. Hyattsville, MD: (2018). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

