Effects of therapeutic zinc supplementation for diarrhea and two preventive zinc supplementation regimens on the incidence and duration of diarrhea and acute respiratory tract infections in rural Laotian children: A randomized controlled trial
- PMID: 32612816
- PMCID: PMC7321011
- DOI: 10.7189/jogh.10.010424
Effects of therapeutic zinc supplementation for diarrhea and two preventive zinc supplementation regimens on the incidence and duration of diarrhea and acute respiratory tract infections in rural Laotian children: A randomized controlled trial
Abstract
Background: Diarrhea and respiratory tract infections are leading causes of childhood morbidity and mortality. This individually randomized, double-blind placebo-controlled trial was designed to evaluate the effects of different zinc supplementation regimens on the incidence and duration of diarrhea and acute lower (ALRI) and upper (AURI) respiratory tract infections among rural Laotian children. The study included 3407 children, 6-23 months at enrollment.
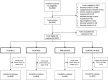
Methods: Children were randomized to one of four study groups: therapeutic zinc supplements for diarrhea treatment (20 mg/d for 10 days with each episode; TZ), daily preventive zinc tablets (7 mg/d; PZ), daily multiple micronutrient powder (10 mg/d zinc, 6 mg/d iron and 13 other micronutrients; MNP), or daily placebo powder for 9 months. Incidence and duration of diarrhea (≥3 liquid stools/24 hours), ALRI (persistent cough with wheezing, stridor or chest in-drawing) and AURI (purulent nasal discharge with cough) were assessed by parental report during weekly home visits and analyzed using negative binomial models.
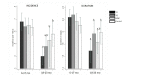
Results: Baseline mean age was 14.2 ± 5.1 months, and 71% had low plasma zinc (<65 μg/dL). Overall diarrhea incidence (0.61 ± 0.01 episodes/100 days at risk) and duration (2.12 ± 0.03 days/episode) did not differ by study group. Age modified the impact of the interventions on diarrhea incidence (P = 0.06) and duration (P = 0.01). In children >18 months, TZ reduced diarrhea incidence by 24% vs MNP (P = 0.035), and 36% vs Control (P = 0.004), but there was no difference with PZ. This patterned remained when analyses were restricted to diarrhea episode occurring after the first treatment with TZ. Also, in children >18 months, TZ reduced diarrhea duration by 15% vs PZ (P = 0.03), and 16% vs Control (P = 0.03), but there was no difference with MNP. There were no overall effects of study group on incidence of ALRI (overall mean 0.005 ± 0.001 episodes/100 days, P = 0.14) or AURI (overall mean 0.09 ± 0.01 episodes/100 days, P = 0.72).
Conclusions: There was no overall impact of TZ, PZ or MNP on diarrhea, ALRI and AURI. However, in children >18 months, TZ significantly reduced both the duration of diarrhea episodes and the incidence of future diarrhea episodes compared with placebo.
Trial registration: ClinicalTrials.gov: NCT02428647.
Copyright © 2020 by the Journal of Global Health. All rights reserved.
Conflict of interest statement
Conflicts of interest: KHB and the spouse of SYH worked for the Bill & Melinda Gates Foundation, which provided part of the financial support. The sponsors had no involvement in the study implementation, data analyses or manuscript writing. All authors have completed the ICMJE uniform disclosure form (available upon request from the corresponding author) and declare no further conflicts of interest.
Figures
References
-
- Fischer Walker CL, Aboubaker S, Van de Weerdt R, Black RE.Zinc for diarrhoea management in Sub-Saharan Africa: a review. East Afr Med J. 2007;84:441-9. - PubMed
