Apomorphine formulation may influence subcutaneous complications from continuous subcutaneous apomorphine infusion in Parkinson's disease
- PMID: 32613445
- PMCID: PMC7578146
- DOI: 10.1007/s00415-020-10031-1
Apomorphine formulation may influence subcutaneous complications from continuous subcutaneous apomorphine infusion in Parkinson's disease
Abstract
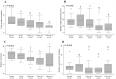
Continuous subcutaneous (s.c.) apomorphine infusion is an effective therapy for Parkinson's disease (PD), but a limitation is the formation of troublesome s.c. nodules. Various chemically non-identical apomorphine formulations are available. Anecdotal experiences have suggested that shifting from one of these (Apo-Go PumpFill®; apoGPF) to another (Apomorphine PharmSwed®; apoPS) may influence the occurrence and severity of s.c. nodules. We, therefore, followed 15 people with advanced PD (median PD-duration, 15 years; median "off"-phase Hoehn and Yahr, IV) on apoGPF and with troublesome s.c. nodules who were switched to apoPS. Data were collected at baseline, at the time of switching, and at a median of 1, 2.5, and 7.3 months post-switch. Total nodule numbers (P < 0.001), size (P < 0.001), consistency (P < 0.001), skin changes (P = 0.058), and pain (P ≤ 0.032) improved over the observation period. PD severity and dyskinesias tended to improve and increase, respectively. Apomorphine doses were stable, but levodopa doses increased by 100 mg/day. Patient-reported apomorphine efficacy tended to increase and all participants remained on apoPS throughout the observation period; with the main patient-reported reason being improved nodules. These observations suggest that patients with s.c. nodules caused by apoGPF may benefit from switching to apoPS in terms of s.c. nodule occurrence and severity. Alternatively, observed benefits may have been due to the switch itself. As nodule formation is a limiting factor in apomorphine treatment, a controlled prospective study comparing local tolerance with different formulations is warranted.
Keywords: Apomorphine; Complications; Nodules; Parkinson’s disease; Safety; Skin.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures
References
-
- Hagell P, Odin P. Apomorphine in Parkinson’s disease. 3. Bremen: UNI-MED Verlag AG; 2014.
-
- Dafsari HS, Martinez-Martin P, Rizos A, Trost M, dos Santos Ghilardi MG, Reddy P, Sauerbier A, Petry-Schmelzer JN, Kramberger M, Borgemeester RWK, Barbe MT, Ashkan K, Silverdale M, Evans J, Odin P, Fonoff ET, Fink GR, Henriksen T, Ebersbach G, Pirtošek Z, Visser-Vandewalle V, Antonini A, Timmermann L, Ray Chaudhuri K. EuroInf 2: subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson’s disease. Mov Disord. 2019;34:353–365. doi: 10.1002/mds.27626. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

