Dermatologic adverse events related to the PI3Kα inhibitor alpelisib (BYL719) in patients with breast cancer
- PMID: 32613539
- PMCID: PMC7398571
- DOI: 10.1007/s10549-020-05726-y
Dermatologic adverse events related to the PI3Kα inhibitor alpelisib (BYL719) in patients with breast cancer
Abstract
Purpose: Rash develops in approximately 50% of patients receiving alpelisib for breast cancer, often requiring dose modifications. Here, we describe the clinicopathologic, laboratory, and management characteristics of alpelisib-related dermatologic adverse events (dAEs).
Methods: A single center-retrospective analysis was conducted. Data were abstracted from electronic medical records.
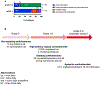
Results: A total of 102 patients (mean age 56 years, range 27-83) receiving alpelisib most frequently in combination with endocrine therapy (79, 77.5%) were included. We identified 41 (40.2%) patients with all-grade rash distributed primarily along the trunk (78%) and extremities (70%) that developed approximately within two weeks of treatment initiation (mean 12.8 ± 1.5 days) and lasted one-week (mean duration 7.1 ± 0.8 days). Of 29 patients with documented morphology of alpelisib-related dAEs, 26 (89.7%) had maculopapular rash. Histology showed perivascular and interface lymphocytic dermatitis. All-grade rash correlated with an increase in serum eosinophils from 2.7 to 4.4%, p < 0.05, and prophylaxis with non-sedating antihistamines (n = 43) was correlated with a reduction of grade 1/2 rash (OR 0.39, p = 0.09). Sixteen (84.2%) of 19 patients with grade 3 dAEs resulted in interruption of alpelisib, which were managed with antihistamines, topical and systemic corticosteroids. We did not observe rash recurrence in 12 (75%) patients who were re-challenged.
Conclusions: A maculopapular rash associated with increased blood eosinophils occurs frequently with alpelisib. While grade 3 rash leads to alpelisib therapy interruption, dermatologic improvement is evident with systemic corticosteroids; and most patients can continue oncologic treatment at a maintained or reduced dose upon re-challenge with alpelisib.
Keywords: Adverse event; Alpelisib; BYL719; PI3K; PIK3CA; Rash.
Conflict of interest statement
Conflict of Interest:
DGW declares no conflicts of interest.
DMB declares no conflicts of interest
VSB declares consulting/advisory agreements with Pfizer and Anthem Foundation. VSB receives research funding from the NIH (5 R37 CA214785).
JFB declares no conflicts of interest.
PRD declares no conflicts of interest.
SAF receives research funding from Genentech/Roche, AstraZeneca, and Decibel Therapeutics. SAF has stock/equity ownership in Urogen Pharma, Allogene Therapeutics, Kronos Bio, Vida Ventures, Kite Pharma, and Neogene Therapeutics.
KLJ declares consulting/advisory board roles with Novartis, Spectrum Pharmaceuticals, ADC Therapeutics, Pfizer, BMS, Abbvie, AstraZeneca, Jounce Therapeutics, Taiho, Oncology, Genentech, Synthon, Lilly Pharmaceuticals, and Intellisphere. KLJ receives research funding from Novartis, Clovis Oncology, Genentech, Astra Zeneca, ADC Therapeutics, Novita Pharmaceuticals, Debio Pharmaceuticals, Pfizer, Lilly Pharmaceuticals, Zymeworks, Immunomedics, and Puma Biotechnology.
DEL declares no conflicts of interest.
TL declares no conflicts of interest.
SM declares consulting/advisory agreements with Daiichi Sankyo, Carrick, Eli Lilly, Genentech, MacroGenics, and GSK Speakers Bureau Genetech. SM receives research support from Novartis, Genentech, Astra Zeneca/ MedImmune, Seattle Genetics, and Daiichi Sankyo.
PR declares a consulting/advisory agreement with Novartis. PR receives institutional research support from Illumina and GRAIL.
MS is an employee of Novartis.
TAT declares consulting/advisory agreements with Genentech/Roche, Pfizer, AstraZeneca, Merck, Puma, Advaxis, Celgene, Innocrin, Genomic Health, Bristol-Myers Squibb, Samsung, Athenex, Aduro Biotech, Halozyme, Daiichi Sankyo, Ionis. TT receives research funding from Eisai, Pfizer, Novartis, Innocrin, AstraZeneca, Astellas, Immunomedics, Genentech, Daiichi, and Carrick.
LTV declares no conflicts of interest.
MEL declares a consulting/advisory agreement with Novartis.
Figures
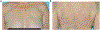
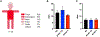
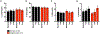
References
-
- Zardavas D, te Marvelde L, Milne RL, Fumagalli D, Fountzilas G, Kotoula V, Razis E, Papaxoinis G, Joensuu H, Moynahan ME, Hennessy BT, Bieche I, Saal LH, Stal O, lacopetta B, Jensen JD, O’Toole S, Lopez-Knowles E, Barbaraeschi M, Noguchi S, Azim HA, Lerma E, Bachelot T, Wang Q, Perez-Tenorio G, can de Velde CJH, Rea DW, Sabine V, Bartlett JMS, Sotiriou C, Michiels S, Loi S (2018) Tumor PIK3CA Genotype and Prognosis in Early-Stage Breast Cancer: A Pooled Analysis of Individual Patient Data. J Clin Oncol 36 (10):981–989. doi: 10.1200/JCO.2017.74.8301 - DOI - PubMed
-
- Razavi P, Chang MT, Xu G, Bandlamudi C, Ross DS, Vasan N, Cai Y, Bielski CM, Donoghue MTA, Jonsson P, Penson A, Shen R, Pareja F, Kundra R, Middha S, Cheng ML, Zehir A, Kandoth C, Patel R, Huberman K, Smyth LM, Jhaveri K, Modi S, Traina TA, Dang C, Zhang W, Weigelt B, Li BT, Ladanyi M, Hyman DM, Schultz N, Robson ME, Hudis C, Brogi E, Viale A, Norton L, Dickler MN, Berger MF, Iacobuzio-Donahue CA, Chandarlapaty S, Scaltriti M, Reis-Filho JS, Solit DB, Taylor BS, Baselga J (2018) The Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers. Cancer Cell 34 (3):427–438. doi:10.1016/j.ccell.2018.08.008 - DOI - PMC - PubMed
-
- Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, Yamashita T, Lu YS, Inoue K, Takahashi M, Papai Z, Longin AS, Mills D, Wilke C, Hirawat S, Juric D, Group S-S (2019) Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med 380 (20):1929–1940. doi:10.1056/NEJMoa1813904 - DOI - PubMed
-
- Juric D, Janku F, Rodon J, Burris HA, Mayer IA, Schuler M, Seggewiss-Bernhardt R, Gil-Martin M, Middleton MR, Baselga J, Bootle D, Demanse D, Blumenstein L, Schumacher K, Huang A, Quadt C, Rugo HS (2019) Alpelisib Plus Fulvestrant in PIK3CA-Altered and PIK3CA-Wild-Type Estrogen Receptor-Positive Advanced Breast Cancer: A Phase 1b Clinical Trial. JAMA Oncol: February 1;5(2):e184475. doi:10.1001/jamaoncol.2018.4475 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

