Trafficking of cholesterol from lipid droplets to mitochondria in bovine luteal cells: Acute control of progesterone synthesis
- PMID: 32614098
- PMCID: PMC7868007
- DOI: 10.1096/fj.202000671R
Trafficking of cholesterol from lipid droplets to mitochondria in bovine luteal cells: Acute control of progesterone synthesis
Abstract
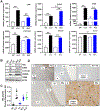
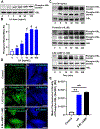
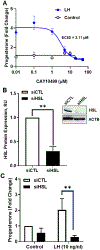
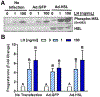
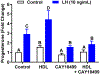
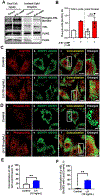
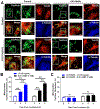
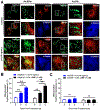
The corpus luteum is a transient endocrine gland that synthesizes and secretes the steroid hormone, progesterone, which is vital for establishment and maintenance of pregnancy. Luteinizing hormone (LH) via activation of protein kinase A (PKA) acutely stimulates luteal progesterone synthesis via a complex process, converting cholesterol via a series of enzymatic reactions, into progesterone. Lipid droplets in steroidogenic luteal cells store cholesterol in the form of cholesterol esters, which are postulated to provide substrate for steroidogenesis. Early enzymatic studies showed that hormone sensitive lipase (HSL) hydrolyzes luteal cholesterol esters. In this study, we tested whether HSL is a critical mediator of the acute actions of LH on luteal progesterone production. Using LH-responsive bovine small luteal cells our results reveal that LH, forskolin, and 8-Br cAMP-induced PKA-dependent phosphorylation of HSL at Ser563 and Ser660, events known to promote HSL activity. Small molecule inhibition of HSL activity and siRNA-mediated knock down of HSL abrogated LH-induced progesterone production. Moreover, western blotting and confocal microscopy revealed that LH stimulates phosphorylation and translocation of HSL to lipid droplets. Furthermore, LH increased trafficking of cholesterol from the lipid droplets to the mitochondria, which was dependent on both PKA and HSL activation. Taken together, these findings identify a PKA/HSL signaling pathway in luteal cells in response to LH and demonstrate the dynamic relationship between PKA, HSL, and lipid droplets in luteal progesterone synthesis.
Keywords: corpus luteum; hormone sensitive lipase; luteinizing hormone; protein kinase A; steroidogenesis.
© 2020 Federation of American Societies for Experimental Biology.
Figures


Similar articles
-
Luteal Lipid Droplets: A Novel Platform for Steroid Synthesis.Endocrinology. 2023 Aug 1;164(9):bqad124. doi: 10.1210/endocr/bqad124. Endocrinology. 2023. PMID: 37586092 Free PMC article.
-
PKA and AMPK Signaling Pathways Differentially Regulate Luteal Steroidogenesis.Endocrinology. 2021 Apr 1;162(4):bqab015. doi: 10.1210/endocr/bqab015. Endocrinology. 2021. PMID: 33502468 Free PMC article.
-
Luteinizing hormone regulates the phosphorylation and localization of the mitochondrial effector dynamin-related protein-1 (DRP1) and steroidogenesis in the bovine corpus luteum.FASEB J. 2020 Apr;34(4):5299-5316. doi: 10.1096/fj.201902958R. Epub 2020 Feb 20. FASEB J. 2020. PMID: 32077149 Free PMC article.
-
Luteinizing Hormone Regulation of Inter-Organelle Communication and Fate of the Corpus Luteum.Int J Mol Sci. 2021 Sep 15;22(18):9972. doi: 10.3390/ijms22189972. Int J Mol Sci. 2021. PMID: 34576135 Free PMC article. Review.
-
Molecular control of luteal secretion of progesterone.Reproduction. 2002 Mar;123(3):333-9. doi: 10.1530/rep.0.1230333. Reproduction. 2002. PMID: 11882010 Review.
Cited by
-
Interferon-tau infusion into the ovine corpus luteum delays luteolysis†.Biol Reprod. 2024 Sep 14;111(3):667-677. doi: 10.1093/biolre/ioae084. Biol Reprod. 2024. PMID: 38869890 Free PMC article.
-
Mechanism of SCD Participation in Lipid Droplet-Mediated Steroidogenesis in Goose Granulosa Cells.Genes (Basel). 2022 Aug 24;13(9):1516. doi: 10.3390/genes13091516. Genes (Basel). 2022. PMID: 36140684 Free PMC article.
-
Unraveling the role of lipid droplets and perilipin 2 in bovine luteal cells.FASEB J. 2024 Jun 15;38(11):e23710. doi: 10.1096/fj.202400260RR. FASEB J. 2024. PMID: 38822676 Free PMC article.
-
Metabolic control of luteinizing hormone-responsive ovarian steroidogenesis.J Biol Chem. 2025 Jan;301(1):108042. doi: 10.1016/j.jbc.2024.108042. Epub 2024 Nov 29. J Biol Chem. 2025. PMID: 39615688 Free PMC article.
-
Luteal Lipid Droplets: A Novel Platform for Steroid Synthesis.Endocrinology. 2023 Aug 1;164(9):bqad124. doi: 10.1210/endocr/bqad124. Endocrinology. 2023. PMID: 37586092 Free PMC article.
References
-
- Daya S, Ward S, Burrows E J. A. j. o. o., and gynecology. (1988) Progesterone profiles in luteal phase defect cycles and outcome of progesterone treatment in patients with recurrent spontaneous abortion. 158, 225–232 - PubMed
-
- Rueda BR, Hendry IR, Hendry WJ III, Stormshak F, Slayden O, and Davis JS (2000) Decreased progesterone levels and progesterone receptor antagonists promote apoptotic cell death in bovine luteal cells. Biology of reproduction 62, 269–276 - PubMed
-
- Holesh JE, and Lord M (2019) Physiology, ovulation In StatPearls [Internet], StatPearls Publishing - PubMed
-
- O’Shea JD, Rodgers RJ, and D’Occhio MJ (1989) Cellular composition of the cyclic corpus luteum of the cow. J Reprod Fertil 85, 483–487 - PubMed
-
- Hansel W, Alila HW, Dowd JP, and Milvae RA (1991) Differential origin and control mechanisms in small and large bovine luteal cells. J Reprod Fertil Suppl 43, 77–89 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

