Characterization of the Cancer Spectrum in Men With Germline BRCA1 and BRCA2 Pathogenic Variants: Results From the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA)
- PMID: 32614418
- PMCID: PMC7333177
- DOI: 10.1001/jamaoncol.2020.2134
Characterization of the Cancer Spectrum in Men With Germline BRCA1 and BRCA2 Pathogenic Variants: Results From the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA)
Erratum in
-
Error in Author Name.JAMA Oncol. 2020 Nov 1;6(11):1815. doi: 10.1001/jamaoncol.2020.4696. JAMA Oncol. 2020. PMID: 32910162 Free PMC article. No abstract available.
Abstract
Importance: The limited data on cancer phenotypes in men with germline BRCA1 and BRCA2 pathogenic variants (PVs) have hampered the development of evidence-based recommendations for early cancer detection and risk reduction in this population.
Objective: To compare the cancer spectrum and frequencies between male BRCA1 and BRCA2 PV carriers.
Design, setting, and participants: Retrospective cohort study of 6902 men, including 3651 BRCA1 and 3251 BRCA2 PV carriers, older than 18 years recruited from cancer genetics clinics from 1966 to 2017 by 53 study groups in 33 countries worldwide collaborating through the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Clinical data and pathologic characteristics were collected.
Main outcomes and measures: BRCA1/2 status was the outcome in a logistic regression, and cancer diagnoses were the independent predictors. All odds ratios (ORs) were adjusted for age, country of origin, and calendar year of the first interview.
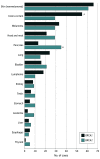
Results: Among the 6902 men in the study (median [range] age, 51.6 [18-100] years), 1634 cancers were diagnosed in 1376 men (19.9%), the majority (922 of 1,376 [67%]) being BRCA2 PV carriers. Being affected by any cancer was associated with a higher probability of being a BRCA2, rather than a BRCA1, PV carrier (OR, 3.23; 95% CI, 2.81-3.70; P < .001), as well as developing 2 (OR, 7.97; 95% CI, 5.47-11.60; P < .001) and 3 (OR, 19.60; 95% CI, 4.64-82.89; P < .001) primary tumors. A higher frequency of breast (OR, 5.47; 95% CI, 4.06-7.37; P < .001) and prostate (OR, 1.39; 95% CI, 1.09-1.78; P = .008) cancers was associated with a higher probability of being a BRCA2 PV carrier. Among cancers other than breast and prostate, pancreatic cancer was associated with a higher probability (OR, 3.00; 95% CI, 1.55-5.81; P = .001) and colorectal cancer with a lower probability (OR, 0.47; 95% CI, 0.29-0.78; P = .003) of being a BRCA2 PV carrier.
Conclusions and relevance: Significant differences in the cancer spectrum were observed in male BRCA2, compared with BRCA1, PV carriers. These data may inform future recommendations for surveillance of BRCA1/2-associated cancers and guide future prospective studies for estimating cancer risks in men with BRCA1/2 PVs.
Conflict of interest statement
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

