Niche-Selective Inhibition of Pathogenic Th17 Cells by Targeting Metabolic Redundancy
- PMID: 32615085
- PMCID: PMC7556360
- DOI: 10.1016/j.cell.2020.06.014
Niche-Selective Inhibition of Pathogenic Th17 Cells by Targeting Metabolic Redundancy
Abstract
Targeting glycolysis has been considered therapeutically intractable owing to its essential housekeeping role. However, the context-dependent requirement for individual glycolytic steps has not been fully explored. We show that CRISPR-mediated targeting of glycolysis in T cells in mice results in global loss of Th17 cells, whereas deficiency of the glycolytic enzyme glucose phosphate isomerase (Gpi1) selectively eliminates inflammatory encephalitogenic and colitogenic Th17 cells, without substantially affecting homeostatic microbiota-specific Th17 cells. In homeostatic Th17 cells, partial blockade of glycolysis upon Gpi1 inactivation was compensated by pentose phosphate pathway flux and increased mitochondrial respiration. In contrast, inflammatory Th17 cells experience a hypoxic microenvironment known to limit mitochondrial respiration, which is incompatible with loss of Gpi1. Our study suggests that inhibiting glycolysis by targeting Gpi1 could be an effective therapeutic strategy with minimum toxicity for Th17-mediated autoimmune diseases, and, more generally, that metabolic redundancies can be exploited for selective targeting of disease processes.
Keywords: CRISPR; EAE; OXPHOS; autoimmunity; colitis; glycolysis; hypoxia; inflammation; metabolic plasticity; segmented filamentous bacteria.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests D.R.L. consults and has equity interest in Chemocentryx, Vedanta, and Pfizer Pharmaceuticals. The NYU School of Medicine has filed a provisional patent application related to this work.
Figures


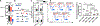


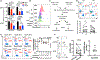
Comment in
-
TH17 cells…Sorting the good out from the bad.Signal Transduct Target Ther. 2020 Sep 19;5(1):207. doi: 10.1038/s41392-020-00316-2. Signal Transduct Target Ther. 2020. PMID: 32951000 Free PMC article. No abstract available.
References
-
- Baronciani L, Zanella A, Bianchi P, Zappa M, Alfinito F, Iolascon A, Tannoia N, Beutler E, and Sirchia G (1996). Study of the molecular defects in glucose phosphate isomerase-deficient patients affected by chronic hemolytic anemia. Blood 88, 2306–2310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

