Qiliqiangxin reduced cardiomyocytes apotosis and improved heart function in infarcted heart through Pink1/Parkin -mediated mitochondrial autophagy
- PMID: 32615967
- PMCID: PMC7330946
- DOI: 10.1186/s12906-020-02992-7
Qiliqiangxin reduced cardiomyocytes apotosis and improved heart function in infarcted heart through Pink1/Parkin -mediated mitochondrial autophagy
Abstract
Background: Qiliqiangxin (QLQX) is a preparation refined from a traditional Chinese medicine compound. It plays an important role in protecting cardiac function after myocardial infarction (MI). However, the underline mechanism of QLQX action is not clear. The purpose of this study was to detect the effects of QLQX on mitophagy after MI.
Methods: Male FVB/NJ mice aged 8-10 weeks were underwent left coronary artery ligation and were orally administered either QLQX (0.25 g/kg/d) or saline. Twenty-eight days after surgical operation, the cardiac function of mice was detected by echocardiography. Electron Microscopy was used to observe the microstructure of cardiomyocytes. Myocardial apoptosis was examined by TdT-mediated dUTP Nick-End Labeling (TUNEL) and western blot. H9c2 cells were cultured in a hypoxic incubator chamber (5% CO2, 1% O2, 94% N2) for 12 h and pretreated with or without QLQX (0.5 mg/mL). The cell apoptosis, reactive oxygen species (ROS), mitochondrial membrane potential and mitophagy were detected.
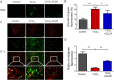
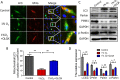
Results: When compared to sham group, the cardiac function of MI mice decreased significantly, and their cardiomyocyte apoptosis and mitochondrial damage were more serious. These MI-induced cardiac changes could be reversed by QLQX treatment. In vitro experiments also confirmed that QLQX could protect cardiomyocytes from hypoxia-induced apoptosis and mitochondrial damage. Further study indicated that QLQX could increase the expression of Pink1 and Parkin in cardiomyocytes.
Conclusion: Qiliqiangxin could reduce cardiomyocytes apotosis and improved heart function in infarcted heart through Pink1-mediated mitochondrial autophagy.
Keywords: Heart function; Mitophagy; Myocardial infarction; Qiliqiangxin.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures




References
-
- Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF, Abbate A. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A. 2011;108:19725–19730. doi: 10.1073/pnas.1108586108. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- FDFR201803/the Free Exploration Project of Hubei University of Medicine
- 2017QDJZR02/the Talent Start -up Fund of Hubei University of Medicine
- 2018GXNSFBA050046/Guangxi Zhuang Autonomous Natural Science Foundation
- 2016ACA157/the Hubei Provincial Technology Innovation Project
- HBMUPI201803/Principal Investigator Grant of Hubei University of Medicine
LinkOut - more resources
Full Text Sources
Medical

