First-in-Human Study of AT13148, a Dual ROCK-AKT Inhibitor in Patients with Solid Tumors
- PMID: 32616501
- PMCID: PMC7611345
- DOI: 10.1158/1078-0432.CCR-20-0700
First-in-Human Study of AT13148, a Dual ROCK-AKT Inhibitor in Patients with Solid Tumors
Abstract
Purpose: AT13148 is an oral AGC kinase inhibitor, which potently inhibits ROCK and AKT kinases. In preclinical models, AT13148 has been shown to have antimetastatic and antiproliferative activity.
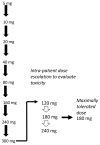
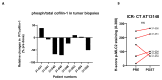
Patients and methods: The trial followed a rolling six design during dose escalation. An intrapatient dose escalation arm to evaluate tolerability and a biopsy cohort to study pharmacodynamic effects were later added. AT13148 was administered orally three days a week (Mon-Wed-Fri) in 28-day cycles. Pharmacokinetic profiles were assessed using mass spectrometry and pharmacodynamic studies included quantifying p-GSK3β levels in platelet-rich plasma (PRP) and p-cofilin and p-MLC2 levels in tumor biopsies.
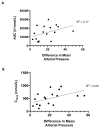
Results: Fifty-one patients were treated on study. The safety of 5-300 mg of AT13148 was studied. Further, the doses of 120-180-240 mg were studied in an intrapatient dose escalation cohort. The dose-limiting toxicities included hypotension (300 mg), pneumonitis, and elevated liver enzymes (240 mg), and skin rash (180 mg). The most common side effects were fatigue, nausea, headaches, and hypotension. On the basis of tolerability, 180 mg was considered the maximally tolerated dose. At 180 mg, mean C max and AUC were 400 nmol/L and 13,000 nmol/L/hour, respectively. At 180 mg, ≥50% reduction of p-cofilin was observed in 3 of 8 posttreatment biopsies.
Conclusions: AT13148 was the first dual potent ROCK-AKT inhibitor to be investigated for the treatment of solid tumors. The narrow therapeutic index and the pharmacokinetic profile led to recommend not developing this compound further. There are significant lessons learned in designing and testing agents that simultaneously inhibit multiple kinases including AGC kinases in cancer.
©2020 American Association for Cancer Research.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- A9098/CRUK_/Cancer Research UK/United Kingdom
- 22897/CRUK_/Cancer Research UK/United Kingdom
- C2739/A22897/CRUK_/Cancer Research UK/United Kingdom
- 24478/CRUK_/Cancer Research UK/United Kingdom
- 11566/CRUK_/Cancer Research UK/United Kingdom
- 9098/CRUK_/Cancer Research UK/United Kingdom
- A11566_4/CRUK_/Cancer Research UK/United Kingdom
- C309/A25144/CRUK_/Cancer Research UK/United Kingdom
- C33043/A24478/CRUK_/Cancer Research UK/United Kingdom
- 11526/CRUK_/Cancer Research UK/United Kingdom
- 20974/CRUK_/Cancer Research UK/United Kingdom
- 28424/CRUK_/Cancer Research UK/United Kingdom
- RP-2016-07-028/DH_/Department of Health/United Kingdom
- C12540/A25128/CRUK_/Cancer Research UK/United Kingdom
- 20740/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

