A phase II single-arm study of pembrolizumab with enzalutamide in men with metastatic castration-resistant prostate cancer progressing on enzalutamide alone
- PMID: 32616555
- PMCID: PMC7333874
- DOI: 10.1136/jitc-2020-000642
A phase II single-arm study of pembrolizumab with enzalutamide in men with metastatic castration-resistant prostate cancer progressing on enzalutamide alone
Abstract
Background: Checkpoint inhibitors can induce profound anticancer responses, but programmed cell death protein-1 (PD-1) inhibition monotherapy has shown minimal activity in prostate cancer. A published report showed that men with prostate cancer who were resistant to the second-generation androgen receptor inhibitor enzalutamide had increased programmed death-ligand 1 (PD-L1) expression on circulating antigen-presenting cells. We hypothesized that the addition of PD-1 inhibition in these patients could induce a meaningful cancer response.
Methods: We evaluated enzalutamide plus the PD-1 inhibitor pembrolizumab in a single-arm phase II study of 28 men with metastatic castration-resistant prostate cancer (mprogressing on enzalutamide alone. Pembrolizumab 200 mg intravenous was given every 3 weeks for four doses with enzalutamide. The primary endpoint was prostate-specific antigen (PSA) decline of ≥50%. Secondary endpoints were objective response, PSA progression-free survival (PFS), time to subsequent treatment, and time to death. Baseline tumor biopsies were obtained when feasible, and samples were sequenced and evaluated for the expression of PD-L1, microsatellite instability (MSI), mutational and neoepitope burdens.
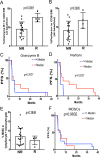
Results: Five (18%) of 28 patients had a PSA decline of ≥50%. Three (25%) of 12 patients with measurable disease at baseline achieved an objective response. Of the five responders, two continue with PSA and radiographic response after 39.3 and 37.8 months. For the entire cohort, median follow-up was 37 months, and median PSA PFS time was 3.8 months (95% CI: 2.8 to 9.9 months). Time to subsequent treatment was 7.21 months (95% CI: 5.1 to 11.1 months). Median overall survival for all patients was 21.9 months (95% CI: 14.7 to 28 .4 months), versus 41.7 months (95% CI: 22.16 to not reached (NR)) in the responders. Of the three responders with baseline biopsies, one had MSI high disease with mutations consistent with DNA-repair defects. None had detectable PD-L1 expression.
Conclusions: Pembrolizumab has activity in mCRPC when added to enzalutamide. Responses were deep and durable and did not require tumor PD-L1 expression or DNA-repair defects.
Trial registration number: clinicaltrials.gov (NCT02312557).
Keywords: biomarkers; combination; drug therapy; immunotherapy; lymphocytes; prostatic neoplasms; tumor; tumor-infiltrating.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JG has received research funding from Astellas/Medivation, Merck, Sanofi, Janssen Biotech, and travel support from Sanofi. CD has received research funding from Bristol Myers Squibb (BMS). He has received consulting fees from BMS, Merck, AstraZeneca (AZ) and Medimmune. He has patents licensed to AZ, BMS and Medimmune. WLR has received research grants, consulting fees, and/or royalties from Bristol-Myers Squibb, Merck, Galectin Therapeutics, and Nektar Therapeutics. TMB has research funding from Astellas/Medivation, Alliance Foundation Trials, Boehringer Ingelheim, Corcept Therapeutics, Endocyte Inc, Janssen Research & Development, OncoGenex, Sotio, and Theraclone Sciences/OncoResponse. TMB receives consulting fees from AbbVie, AstraZeneca, Astellas Pharma, Bayer, Boehringer Ingelheim, Clovis Oncology, GlaxoSmithKline, Janssen Biotech, Janssen Japan, Merck, and Pfizer and has stock ownership in Salarius Pharmaceuticals, and Arvinas Inc. JJA, GVT, and RS have no conflicts. RCB is a co-owner of Third Coast Therapeutics Inc, which has an option to license patents of which he is an owner. AEM has a sponsored research agreement with AstraZeneca and has received reagents from Genetech. JJA has received consulting fees from Merck.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
