Variability and sex-dependence of hypothermic neuroprotection in a rat model of neonatal hypoxic-ischaemic brain injury: a single laboratory meta-analysis
- PMID: 32616806
- PMCID: PMC7331720
- DOI: 10.1038/s41598-020-67532-2
Variability and sex-dependence of hypothermic neuroprotection in a rat model of neonatal hypoxic-ischaemic brain injury: a single laboratory meta-analysis
Abstract
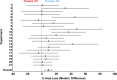
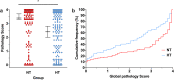
Therapeutic hypothermia (HT) is standard care for term infants with hypoxic-ischaemic (HI) encephalopathy. However, the efficacy of HT in preclinical models, such as the Vannucci model of unilateral HI in the newborn rat, is often greater than that reported from clinical trials. Here, we report a meta-analysis of data from every experiment in a single laboratory, including pilot data, examining the effect of HT in the Vannucci model. Across 21 experiments using 106 litters, median (95% CI) hemispheric area loss was 50.1% (46.0-51.9%; n = 305) in the normothermia group, and 41.3% (35.1-44.9%; n = 317) in the HT group, with a bimodal injury distribution. Median neuroprotection by HT was 17.6% (6.8-28.3%), including in severe injury, but was highly-variable across experiments. Neuroprotection was significant in females (p < 0.001), with a non-significant benefit in males (p = 0.07). Animals representing the median injury in each group within each litter (n = 277, 44.5%) were also analysed using formal neuropathology, which showed neuroprotection by HT throughout the brain, particularly in females. Our results suggest an inherent variability and sex-dependence of the neuroprotective response to HT, with the majority of studies in the Vannucci model vastly underpowered to detect true treatment effects due to the distribution of injury.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Shankaran S, Laptook AR, Pappas A, McDonald SA, Das A, Tyson JE, Poindexter BB, Schibler K, Bell EF, Heyne RJ, Pedroza C, Bara R, Van Meurs KP, Huitema CMP, Grisby C, Devaskar U, Ehrenkranz RA, Harmon HM, Chalak LF, DeMauro SB, Garg M, Hartley-McAndrew ME, Khan AM, Walsh MC, Ambalavanan N, Brumbaugh JE, Watterberg KL, Shepherd EG, Hamrick SEG, Barks J, Cotten CM, Kilbride HW, Higgins RD, Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic–ischemic encephalopathy: a randomized clinical trial. Jama. 2017;318(1):57–67. doi: 10.1001/jama.2017.7218. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

