Cu-Catalyzed Hydroboration of Benzylidenecyclopropanes: Reaction Optimization, (Hetero)Aryl Scope, and Origins of Pathway Selectivity
- PMID: 32617185
- PMCID: PMC7331956
- DOI: 10.1021/acscatal.9b03557
Cu-Catalyzed Hydroboration of Benzylidenecyclopropanes: Reaction Optimization, (Hetero)Aryl Scope, and Origins of Pathway Selectivity
Abstract
The copper-catalyzed hydroboration of benzylidenecyclopropanes, conveniently accessed in one step from readily available benzaldehydes, is reported. Under otherwise identical reaction conditions, two distinct phosphine ligands grant access to different products by either suppressing or promoting cyclopropane opening via β-carbon elimination. Computational studies provide insight into how the rigidity and steric environment of these different bis-phosphine ligands influence the relative activation energies of β-carbon elimination versus protodecupration from the key benzylcopper intermediate. The method tolerates a wide variety of heterocycles prevalent in clinical and pre-clinical drug development, giving access to valuable synthetic intermediates. The versatility of the tertiary cyclopropylboronic ester products is demonstrated through several derivatization reactions.
Keywords: Copper catalysis; benzylidenecyclopropanes; cyclopropylboronic esters; heterocycles; hydroborations; β-carbon elimination.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
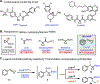


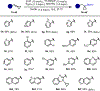




References
-
- de Meijere A; Kozhushkov SI; Schill H Three-Membered-Ring-Based Molecular Architectures. Chem. Rev 2006, 106, 4926–4996. - PubMed
- Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem 2009, 52, 6752–6756. - PubMed
- Chen DY-K; Pouwer RH; Richard J-A Recent Advances in the Total Synthesis of Cyclopropane-Containing Natural Products. Chem. Soc. Rev 2012, 41, 4631–4642. - PubMed
- Taylor RD; MacCoss M; Lawson ADG Rings in Drugs. J. Med. Chem 2014, 57, 5845–5859. - PubMed
-
- Gagnon A; Duplessis M; Fader L Arylcyclopropanes: Properties, Synthesis and Use in Medicinal Chemistry. Org. Prep. Proced. Int 2010, 42, 1–69.
- Talele TT The “Cyclopropyl Fragment” is a Versatile Player that Frequently Appears in Preclinical/Clinical Drug Molecules. J. Med. Chem 2016, 59, 8712–8756. - PubMed
- Derosa J; O’Duill ML; Holcomb M; Boulous MN; Patman RL; Wang F; Tran-Dube M; McAlpine I; Engle KM Copper-Catalyzed Chan−Lam Cyclopropylation of Phenols and Azaheterocycles. J. Org. Chem 2018, 83, 3417–3425. - PMC - PubMed
-
- Butcher KJ; Denton SM; Field SE; Gillmore AT; Harbottle GW; Howard RM; Laity DA; Ngono CJ; Pibworth BA Convergent Asymmetric Synthesis of Two Complex TRPV1 Antagonists. Org. Process Res. Dev 2011, 15, 1192–1200.
-
- Micheli F; Cavanni P; Andreotti D; Arban R; Benedetti R; Bertani B; Bettati M; Bettelini L; Bonanomi G; Braggio S; Carletti R; Checchia A; Corsi M; Fazzolari E; Fontana S; Marchioro C; Merlo-Pich E; Negri M; Oliosi B; Ratti E; Read KD; Roscic M; Sartori I; Spada S; Tedesco G; Tarsi L; Terreni S; Visentini F; Zocchi A; Zonzini L; Di Fabio R 6-(3,4-Dichlorophenyl)-1-[(methyloxy)methyl]-3-azabicyclo[4.1.0]heptane: A New Potent and Selective Triple Reuptake Inhibitor. J. Med. Chem 2010, 53, 4989–5001. - PubMed
-
- Zillhardt M; Park S-M; Romero IL; Sawada K; Montag A; Krausz T; Yamada SD; Peter ME; Lengyel E Foretinib (GSK1363089), an Orally Available Multikinase Inhibitor of c-Met and VEGFR-2, Blocks Proliferation, Induces Anoikis, and Impairs Ovarian Cancer Metastasis. Clin. Cancer Res 2011, 17, 4042–4051. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
