High neutralizing antibody titer in intensive care unit patients with COVID-19
- PMID: 32618497
- PMCID: PMC7473321
- DOI: 10.1080/22221751.2020.1791738
High neutralizing antibody titer in intensive care unit patients with COVID-19
Abstract
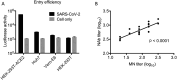
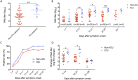
Coronavirus disease 2019 (COVID-19) has a wide spectrum of disease severity from mild upper respiratory symptoms to respiratory failure. The role of neutralizing antibody (NAb) response in disease progression remains elusive. This study determined the seroprevalence of 733 non-COVID-19 individuals from April 2018 to February 2020 in the Hong Kong Special Administrative Region and compared the neutralizing antibody (NAb) responses of eight COVID-19 patients admitted to the intensive care unit (ICU) with those of 42 patients not admitted to the ICU. We found that NAb against SARS-CoV-2 was not detectable in any of the anonymous serum specimens from the 733 non-COVID-19 individuals. The peak serum geometric mean NAb titer was significantly higher among the eight ICU patients than the 42 non-ICU patients (7280 [95% confidence interval (CI) 1468-36099]) vs (671 [95% CI, 368-1223]). Furthermore, NAb titer increased significantly at earlier infection stages among ICU patients than among non-ICU patients. The median number of days to reach the peak Nab titers after symptoms onset was shorter among the ICU patients (17.6) than that of the non-ICU patients (20.1). Multivariate analysis showed that oxygen requirement and fever during admission were the only clinical factors independently associated with higher NAb titers. Our data suggested that SARS-CoV-2 was unlikely to have silently spread before the COVID-19 emergence in Hong Kong. ICU patients had an accelerated and augmented NAb response compared to non-ICU patients, which was associated with disease severity. Further studies are required to understand the relationship between high NAb response and disease severity.
Keywords: COVID19; ICU patient; SARS-CoV-2; disease severity; neutralizing antibody.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- Zhou J, Li C, Liu X, et al. . Infection of bat and human intestinal organoids by SARS-CoV-2. Nat Med. 2020. Accepted and in press. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
