Inhibition of APE1/Ref-1 Redox Signaling Alleviates Intestinal Dysfunction and Damage to Myenteric Neurons in a Mouse Model of Spontaneous Chronic Colitis
- PMID: 32618996
- PMCID: PMC8287929
- DOI: 10.1093/ibd/izaa161
Inhibition of APE1/Ref-1 Redox Signaling Alleviates Intestinal Dysfunction and Damage to Myenteric Neurons in a Mouse Model of Spontaneous Chronic Colitis
Abstract
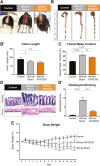
Background: Inflammatory bowel disease (IBD) associates with damage to the enteric nervous system (ENS), leading to gastrointestinal (GI) dysfunction. Oxidative stress is important for the pathophysiology of inflammation-induced enteric neuropathy and GI dysfunction. Apurinic/apyrimidinic endonuclease 1/redox factor-1 (APE1/Ref-1) is a dual functioning protein that is an essential regulator of the cellular response to oxidative stress. In this study, we aimed to determine whether an APE1/Ref-1 redox domain inhibitor, APX3330, alleviates inflammation-induced oxidative stress that leads to enteric neuropathy in the Winnie murine model of spontaneous chronic colitis.
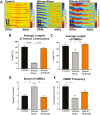
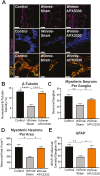
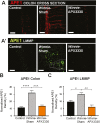
Methods: Winnie mice received APX3330 or vehicle via intraperitoneal injections over 2 weeks and were compared with C57BL/6 controls. In vivo disease activity and GI transit were evaluated. Ex vivo experiments were performed to assess functional parameters of colonic motility, immune cell infiltration, and changes to the ENS.
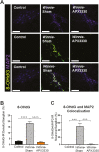
Results: Targeting APE1/Ref-1 redox activity with APX3330 improved disease severity, reduced immune cell infiltration, restored GI function ,and provided neuroprotective effects to the enteric nervous system. Inhibition of APE1/Ref-1 redox signaling leading to reduced mitochondrial superoxide production, oxidative DNA damage, and translocation of high mobility group box 1 protein (HMGB1) was involved in neuroprotective effects of APX3330 in enteric neurons.
Conclusions: This study is the first to investigate inhibition of APE1/Ref-1's redox activity via APX3330 in an animal model of chronic intestinal inflammation. Inhibition of the redox function of APE1/Ref-1 is a novel strategy that might lead to a possible application of APX3330 for the treatment of IBD.
Keywords: APE1/Ref-1; APX3330; DNA damage; IBD; chronic intestinal inflammation; enteric nervous system; oxidative stress.
© 2020 Crohn’s & Colitis Foundation. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

