Pathogen reduction of double-dose platelet concentrates from pools of eight buffy coats: Product quality, safety, and economic aspects
- PMID: 32619068
- PMCID: PMC7540585
- DOI: 10.1111/trf.15926
Pathogen reduction of double-dose platelet concentrates from pools of eight buffy coats: Product quality, safety, and economic aspects
Abstract
Background: Pathogen reduction (PR) of platelet concentrates (PCs) contributes to the safety of platelet (PLT) transfusion by reducing the risk of transfusion-transmitted infections and transfusion-associated graft-versus-host disease. In vitro quality of pathogen-reduced double-dose PC (PR-PC) made of eight whole blood (WB)-derived buffy coats (BCs) were evaluated.
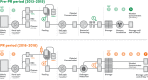
Methods: Eight small-volume WB BCs from donors with at least 200 × 109 PLT/L were pooled with an additive solution to produce double-dose PCs (DD-PCs), which were treated with amotosalen/ultraviolet A light in a dual storage processing set, yielding 2 units of PR-PC. Quality controls were undertaken as per European Directive for the Quality of Medicines (EDQM) guidelines. PLT recovery rates were measured. Production costs and savings were compared over the 3 years before and after PR implementation.
Results: In the pre-PR period, 19 666 PCs were produced, compared to 17 307 PCs in the PR period. Single BC in the PR period had 41 ± 2 mL, hematocrit 0.39 ± 0.04 and 1.06 ± 0.18 × 1011 PLTs, and showed a recovery of 91% ± 8%. After pooling, separation, PR treatment of DD-PC, and splitting, each single PC had 189 ± 6 mL with 2.52 ± 0.34 × 1011 PLTs, compared to 2.48 ± 0.40 in the pre-PR period. The PLT recovery rate after PR was 87% ± 14%. EDQM requirements were met. An increase of about €12 (+7.5%) per PC from the pre-PR to the PR period was identified.
Conclusion: A new production method resulting in two PR-PCs made from pools of 8 BCs with use of one PR set was successfully introduced, and our experience of nearly 3 years demonstrated the high efficacy and in vitro quality of the PR-PCs obtained.
© 2020 The Authors. Transfusion published by Wiley Periodicals LLC. on behalf of AABB.
Conflict of interest statement
The Graz Blood Center performs company‐funded trials for Cerus and is under contract to perform routine PR for platelets and plasma in. KR and WH declare no conflicts of interest. PS has received honorarium and reinbursement for travel expenses in the past 5 years.
Figures


References
-
- Kwon SY, Kim IS, Bae JE, et al. Pathogen inactivation efficacy of Mirasol PRT system and intercept blood system for non‐leucoreduced platelet‐rich plasma‐derived platelets suspended in plasma. Vox Sang. 2014;107:254–260. - PubMed
-
- Hechler B, Ohlmann P, Chafey P, et al. Preserved functional and biochemical characteristics of platelet components prepared with amotosalen and ultraviolet A for pathogen inactivation. Transfusion. 2013;53:1187–1200. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

