Bi-allelic DNAH8 Variants Lead to Multiple Morphological Abnormalities of the Sperm Flagella and Primary Male Infertility
- PMID: 32619401
- PMCID: PMC7413861
- DOI: 10.1016/j.ajhg.2020.06.004
Bi-allelic DNAH8 Variants Lead to Multiple Morphological Abnormalities of the Sperm Flagella and Primary Male Infertility
Abstract
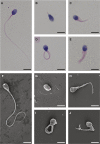
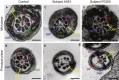
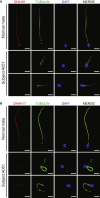
Sperm malformation is a direct factor for male infertility. Multiple morphological abnormalities of the flagella (MMAF), a severe form of asthenoteratozoospermia, are characterized by immotile spermatozoa with malformed and/or absent flagella in the ejaculate. Previous studies indicated genetic heterogeneity in MMAF. To further define genetic factors underlying MMAF, we performed whole-exome sequencing in a cohort of 90 Chinese MMAF-affected men. Two cases (2.2%) were identified as carrying bi-allelic missense DNAH8 variants, variants which were either absent or rare in the control human population and were predicted to be deleterious by multiple bioinformatic tools. Re-analysis of exome data from a second cohort of 167 MMAF-affected men from France, Iran, and North Africa permitted the identification of an additional male carrying a DNAH8 homozygous frameshift variant. DNAH8 encodes a dynein axonemal heavy-chain component that is expressed preferentially in the testis. Hematoxylin-eosin staining and electron microscopy analyses of the spermatozoa from men harboring bi-allelic DNAH8 variants showed a highly aberrant morphology and ultrastructure of the sperm flagella. Immunofluorescence assays performed on the spermatozoa from men harboring bi-allelic DNAH8 variants revealed the absent or markedly reduced staining of DNAH8 and its associated protein DNAH17. Dnah8-knockout male mice also presented typical MMAF phenotypes and sterility. Interestingly, intracytoplasmic sperm injections using the spermatozoa from Dnah8-knockout male mice resulted in good pregnancy outcomes. Collectively, our experimental observations from humans and mice demonstrate that DNAH8 is essential for sperm flagellar formation and that bi-allelic deleterious DNAH8 variants lead to male infertility with MMAF.
Keywords: CRISPR; DNAH17; DNAH8; ICSI; dynein; exome; flagella; infertility; knockout; sperm.
Copyright © 2020 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Bi-allelic Loss-of-function Variants in CFAP58 Cause Flagellar Axoneme and Mitochondrial Sheath Defects and Asthenoteratozoospermia in Humans and Mice.Am J Hum Genet. 2020 Sep 3;107(3):514-526. doi: 10.1016/j.ajhg.2020.07.010. Epub 2020 Aug 12. Am J Hum Genet. 2020. PMID: 32791035 Free PMC article.
-
Bi-allelic mutations of DNAH10 cause primary male infertility with asthenoteratozoospermia in humans and mice.Am J Hum Genet. 2021 Aug 5;108(8):1466-1477. doi: 10.1016/j.ajhg.2021.06.010. Epub 2021 Jul 7. Am J Hum Genet. 2021. PMID: 34237282 Free PMC article.
-
Novel bi-allelic variants in DNAH2 cause severe asthenoteratozoospermia with multiple morphological abnormalities of the flagella.Reprod Biomed Online. 2021 May;42(5):963-972. doi: 10.1016/j.rbmo.2021.01.011. Epub 2021 Jan 22. Reprod Biomed Online. 2021. PMID: 33771466
-
Novel mutations in DNAH17 cause sperm flagellum defects and their influence on ICSI outcome.J Assist Reprod Genet. 2023 Oct;40(10):2485-2492. doi: 10.1007/s10815-023-02897-7. Epub 2023 Aug 14. J Assist Reprod Genet. 2023. PMID: 37574497 Free PMC article. Review.
-
A novel variant in CFAP69 causes asthenoteratozoospermia with treatable ART outcomes and a literature review.J Assist Reprod Genet. 2023 Sep;40(9):2175-2184. doi: 10.1007/s10815-023-02873-1. Epub 2023 Jul 1. J Assist Reprod Genet. 2023. PMID: 37392306 Free PMC article. Review.
Cited by
-
Whole exome sequencing analysis of 167 men with primary infertility.BMC Med Genomics. 2024 Sep 12;17(1):230. doi: 10.1186/s12920-024-02005-3. BMC Med Genomics. 2024. PMID: 39267058 Free PMC article.
-
Identification of CFAP52 as a novel diagnostic target of male infertility with defects of sperm head-tail connection and flagella development.Elife. 2023 Dec 21;12:RP92769. doi: 10.7554/eLife.92769. Elife. 2023. PMID: 38126872 Free PMC article.
-
Deficiency of primate-specific SSX1 induced asthenoteratozoospermia in infertile men and cynomolgus monkey and tree shrew models.Am J Hum Genet. 2023 Mar 2;110(3):516-530. doi: 10.1016/j.ajhg.2023.01.016. Epub 2023 Feb 15. Am J Hum Genet. 2023. PMID: 36796361 Free PMC article.
-
Deleterious variants in TAF7L cause human oligoasthenoteratozoospermia and its impairing histone to protamine exchange inducing reduced in vitro fertilization.Front Endocrinol (Lausanne). 2023 Jan 11;13:1099270. doi: 10.3389/fendo.2022.1099270. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36714566 Free PMC article.
-
Loss of DRC1 function leads to multiple morphological abnormalities of the sperm flagella and male infertility in human and mouse.Hum Mol Genet. 2021 Oct 13;30(21):1996-2011. doi: 10.1093/hmg/ddab171. Hum Mol Genet. 2021. PMID: 34169321 Free PMC article.
References
-
- Hosseini B., Nourmohamadi M., Hajipour S., Taghizadeh M., Asemi Z., Keshavarz S.A., Jafarnejad S. The Effect of Omega-3 Fatty Acids, EPA, and/or DHA on Male Infertility: A Systematic Review and Meta-analysis. J. Diet. Suppl. 2019;16:245–256. - PubMed
-
- Ben Khelifa M., Coutton C., Zouari R., Karaouzène T., Rendu J., Bidart M., Yassine S., Pierre V., Delaroche J., Hennebicq S. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am. J. Hum. Genet. 2014;94:95–104. - PMC - PubMed
-
- Baccetti B., Collodel G., Estenoz M., Manca D., Moretti E., Piomboni P. Gene deletions in an infertile man with sperm fibrous sheath dysplasia. Hum. Reprod. 2005;20:2790–2794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

