Structural Insights into Pseudokinase Domains of Receptor Tyrosine Kinases
- PMID: 32619402
- PMCID: PMC7543951
- DOI: 10.1016/j.molcel.2020.06.018
Structural Insights into Pseudokinase Domains of Receptor Tyrosine Kinases
Abstract
Despite their apparent lack of catalytic activity, pseudokinases are essential signaling molecules. Here, we describe the structural and dynamic properties of pseudokinase domains from the Wnt-binding receptor tyrosine kinases (PTK7, ROR1, ROR2, and RYK), which play important roles in development. We determined structures of all pseudokinase domains in this family and found that they share a conserved inactive conformation in their activation loop that resembles the autoinhibited insulin receptor kinase (IRK). They also have inaccessible ATP-binding pockets, occluded by aromatic residues that mimic a cofactor-bound state. Structural comparisons revealed significant domain plasticity and alternative interactions that substitute for absent conserved motifs. The pseudokinases also showed dynamic properties that were strikingly similar to those of IRK. Despite the inaccessible ATP site, screening identified ATP-competitive type-II inhibitors for ROR1. Our results set the stage for an emerging therapeutic modality of "conformational disruptors" to inhibit or modulate non-catalytic functions of pseudokinases deregulated in disease.
Keywords: GZD824; Wnt signaling; cancer; growth factor signaling; ponatinib; protein conformation; pseudokinases; receptor tyrosine kinases; targeted therapies.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures

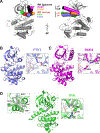





References
-
- Ablooglu AJ, Frankel M, Rusinova E, Ross JB, and Kohanski RA (2001). Multiple activation loop conformations and their regulatory properties in the insulin receptor’s kinase domain. J. Biol. Chem. 276, 46933–46940. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

