A Contaminant Impurity, Not Rigosertib, Is a Tubulin Binding Agent
- PMID: 32619468
- PMCID: PMC9648421
- DOI: 10.1016/j.molcel.2020.05.024
A Contaminant Impurity, Not Rigosertib, Is a Tubulin Binding Agent
Abstract
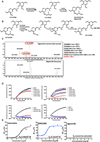
Rigosertib is a styryl benzyl sulfone that inhibits growth of tumor cells and acts as a RAS mimetic by binding to Ras binding domains of RAS effectors. A recent study attributed rigosertib's mechanism of action to microtubule binding. In that study, rigosertib was obtained from a commercial vendor. We compared the purity of clinical-grade and commercially sourced rigosertib and found that commercially sourced rigosertib contains approximately 5% ON01500, a potent inhibitor of tubulin polymerization. Clinical-grade rigosertib, which is free of this impurity, does not exhibit tubulin-binding activity. Cell lines expressing mutant β-tubulin have also been reported to be resistant to rigosertib. However, our study showed that these cells failed to proliferate in the presence of rigosertib at concentrations that are lethal to wild-type cells. Rigosertib induced a senescence-like phenotype in the small percentage of surviving cells, which could be incorrectly scored as resistant using short-term cultures.
Keywords: ON01500; ON01910; RAS; Ras binding domain; rigosertib; tubulin polymerization.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests E.P.R. is an equity holder, board member, and paid consultant of Onconova Therapeutics. S.J.B. is a paid consultant of Onconova Therapeutics. M.V.R.R. and S.C.C. are stockholders and paid consultants of Onconova Therapeutics, Inc. M.V.R.R. and E.P.R. are named inventors on pending and/or issued patents filed by Temple University.
Figures



References
-
- Agoni L, Basu I, Gupta S, Alfieri A, Gambino A, Goldberg GL, Reddy EP, and Guha C (2014). Rigosertib is a more effective radiosensitizer than cisplatin in concurrent chemoradiation treatment of cervical carcinoma, in vitro and in vivo. Int. J. Radiat. Oncol. Biol. Phys. 88, 1180–1187. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

