Input Connectivity Reveals Additional Heterogeneity of Dopaminergic Reinforcement in Drosophila
- PMID: 32619479
- PMCID: PMC7443709
- DOI: 10.1016/j.cub.2020.05.077
Input Connectivity Reveals Additional Heterogeneity of Dopaminergic Reinforcement in Drosophila
Abstract
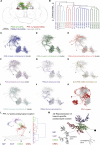
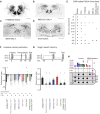
Different types of Drosophila dopaminergic neurons (DANs) reinforce memories of unique valence and provide state-dependent motivational control [1]. Prior studies suggest that the compartment architecture of the mushroom body (MB) is the relevant resolution for distinct DAN functions [2, 3]. Here we used a recent electron microscope volume of the fly brain [4] to reconstruct the fine anatomy of individual DANs within three MB compartments. We find the 20 DANs of the γ5 compartment, at least some of which provide reward teaching signals, can be clustered into 5 anatomical subtypes that innervate different regions within γ5. Reconstructing 821 upstream neurons reveals input selectivity, supporting the functional relevance of DAN sub-classification. Only one PAM-γ5 DAN subtype γ5(fb) receives direct recurrent feedback from γ5β'2a mushroom body output neurons (MBONs) and behavioral experiments distinguish a role for these DANs in memory revaluation from those reinforcing sugar memory. Other DAN subtypes receive major, and potentially reinforcing, inputs from putative gustatory interneurons or lateral horn neurons, which can also relay indirect feedback from MBONs. We similarly reconstructed the single aversively reinforcing PPL1-γ1pedc DAN. The γ1pedc DAN inputs mostly differ from those of γ5 DANs and they cluster onto distinct dendritic branches, presumably separating its established roles in aversive reinforcement and appetitive motivation [5, 6]. Tracing also identified neurons that provide broad input to γ5, β'2a, and γ1pedc DANs, suggesting that distributed DAN populations can be coordinately regulated. These connectomic and behavioral analyses therefore reveal further complexity of dopaminergic reinforcement circuits between and within MB compartments.
Keywords: Drosophila; connectomics; dopamine; extinction; learning; memory; mushroom body; reward.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures


Comment in
-
Connectomics: Bringing Fly Neural Circuits into Focus.Curr Biol. 2020 Aug 17;30(16):R944-R947. doi: 10.1016/j.cub.2020.06.068. Curr Biol. 2020. PMID: 32810456
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

