Analysis of adjunctive serological detection to nucleic acid test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection diagnosis
- PMID: 32619956
- PMCID: PMC7318959
- DOI: 10.1016/j.intimp.2020.106746
Analysis of adjunctive serological detection to nucleic acid test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection diagnosis
Abstract
Background: The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused coronavirus disease 2019 (COVID-19) epidemic in China, December 2019. The clinical features and treatment of COVID-19 patients remain largely elusive. However, accurate detection is required for SARS-CoV-2 infection diagnosis. We aimed to evaluate the antibodies-based test and nucleic acid-based test for SARS-CoV-2-infected patients.
Methods: We retrospectively studied 133 patients diagnosed with SARS-CoV-2 and admitted to Renmin Hospital of Wuhan University, China, from January 23 to March 1, 2020. Demographic data, clinical records, laboratory tests, and outcomes were collected. Data were accessed by SARS-CoV-2 IgM-IgG antibody test and real-time reverse transcriptase PCR (RT-PCR) detection for SARS-CoV-2 nucleic acid in COVID-19 patients.
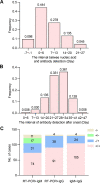
Results: Of 133 COVID-19 patients, there were 44 moderate cases, 52 severe cases, and 37 critical cases with no differences in gender and age among three subgroups. In RT-PCR detection, the positive rate was 65.9%, 71.2%, and 67.6% in moderate, severe, and critical cases, respectively. Whereas the positive rate of IgM/IgG antibody detection in patients was 79.5%/93.2%, 82.7%/100%, and 73.0%/97.3% in moderate, severe, and critical cases, respectively. Moreover, the IgM and IgG antibodies concentrations were also examined with no differences among three subgroups.
Conclusion: The IgM-IgG antibody test exhibited a useful adjunct to RT-PCR detection, and improved the accuracy in COVID-19 diagnosis regardless of the severity of illness, which provides an effective complement to the false-negative results from a nucleic acid test for SARS-CoV-2 infection diagnosis after onsets.
Keywords: COVID-19; IgM-IgG antibody test; Nucleic acid test; SARS-CoV-2; Severity of illness.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- World Health Organization (WHO), Coronavirus diseases (COVID-19): Situation reports – 138, 2020.
-
- X. Yang, Y. Yu, J. Xu, H. Shu, J. Xia, H. Liu, Y. Wu, L. Zhang, Z. Yu, M. Fang, T. Yu, Y. Wang, S. Pan, X. Zou, S. Yuan, Y. Shang, Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study, The Lancet. Respiratory Med. (2020). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

