New Twists in Detecting mRNA Modification Dynamics
- PMID: 32620324
- PMCID: PMC7326690
- DOI: 10.1016/j.tibtech.2020.06.002
New Twists in Detecting mRNA Modification Dynamics
Abstract
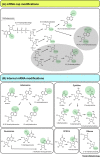
Modified nucleotides in mRNA are an essential addition to the standard genetic code of four nucleotides in animals, plants, and their viruses. The emerging field of epitranscriptomics examines nucleotide modifications in mRNA and their impact on gene expression. The low abundance of nucleotide modifications and technical limitations, however, have hampered systematic analysis of their occurrence and functions. Selective chemical and immunological identification of modified nucleotides has revealed global candidate topology maps for many modifications in mRNA, but further technical advances to increase confidence will be necessary. Single-molecule sequencing introduced by Oxford Nanopore now promises to overcome such limitations, and we summarize current progress with a particular focus on the bioinformatic challenges of this novel sequencing technology.
Keywords: 2′-O-ribose methylation; Nanopore; m(5)C; m(6)A; m(6)Am; mRNA modifications.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

References
-
- Soller M., Fray R. RNA modifications in gene expression control. Biochim. Biophys. Acta Gene Regul. Mech. 2019;1862:219–221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

