Reorganisation of faecal microbiota transplant services during the COVID-19 pandemic
- PMID: 32620549
- PMCID: PMC7456726
- DOI: 10.1136/gutjnl-2020-321829
Reorganisation of faecal microbiota transplant services during the COVID-19 pandemic
Abstract
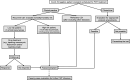
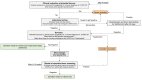
The COVID-19 pandemic has led to an exponential increase in SARS-CoV-2 infections and associated deaths, and represents a significant challenge to healthcare professionals and facilities. Individual countries have taken several prevention and containment actions to control the spread of infection, including measures to guarantee safety of both healthcare professionals and patients who are at increased risk of infection from COVID-19. Faecal microbiota transplantation (FMT) has a well-established role in the treatment of Clostridioides difficile infection. In the time of the pandemic, FMT centres and stool banks are required to adopt a workflow that continues to ensure reliable patient access to FMT while maintaining safety and quality of procedures. In this position paper, based on the best available evidence, worldwide FMT experts provide guidance on issues relating to the impact of COVID-19 on FMT, including patient selection, donor recruitment and selection, stool manufacturing, FMT procedures, patient follow-up and research activities.
Keywords: colonic microflora; diarrhoeal disease.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AG reports personal fees for consultancy from Eisai Srl, 3PSolutions, Real Time Meeting, Fondazione Istituto Danone, Sinergie Srl, Board MRGE and Sanofi SpA personal fees for acting as a speaker for Takeda SpA, AbbVie and Sandoz SpA and personal fees for acting on advisory boards for VSL3 and Eisai. BHM reports personal fees from Finch Therapeutics Group. CRK has served as a clinical advisor, with no financial compensation, for OpenBiome since 2013; she is a local principal investigator for the PRISM-3 clinical trial, for which her institution receives some salary support for a research coordinator and compensation from Finch Therapeutics Group for each patient enrolled. FZ reports grants from the non-profit China Microbiota Transplantation System (fmtBank) and has a patent for GenFMTer for separating microbiota issued to FMT medical. GC has received personal fees for acting as advisor for Ferring Therapeutics. GI has received personal fees for acting as speaker from Biocodex, Danone, Metagenics, and for acting as consultant/advisor from Ferring Therapeutics, Giuliani, Metagenics. HS reports personal fees from Danone, Enterome, Takeda, AbbVie, Roche, Amgen, Danone, BiomX, Ferring, BMS, Astellas, MSD, Novartis, Tillotts Pharma, and Biose, and grants from Biocodex, Danone and BiomX, and is a co-founder of Exeliom Biosciences. JJK and EJK report grants from Vedanta Biosciences. JRA reports personal fees from Finch Therapeutics and has a non-financial relationship with OpenBiome as a scientific advisor. MF reports personal fees from Finch Therapeutics Group, Rebiotix, Takeda, AbbVie and Janssen. SCN reports grants from Ferring and personal fees from Takeda, AbbVie, Janssen and Tillotts. SPC reports non-financial support from Janssen and personal fees from Shire, Ferring, Microbiotica and Pfizer. ZK is an employee and shareholder of Finch Therapeutics and is an unpaid special advisor for OpenBiome.
Figures
Comment in
-
Enhanced donor screening for faecal microbial transplantation during COVID-19.Gut. 2021 Nov;70(11):2219-2220. doi: 10.1136/gutjnl-2021-324593. Epub 2021 Mar 31. Gut. 2021. PMID: 33789964 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous